Elegant Engineering



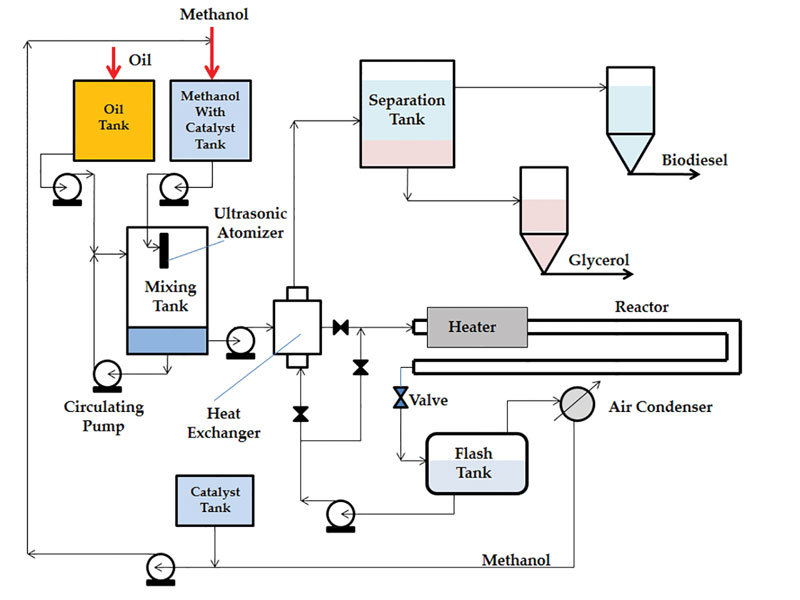
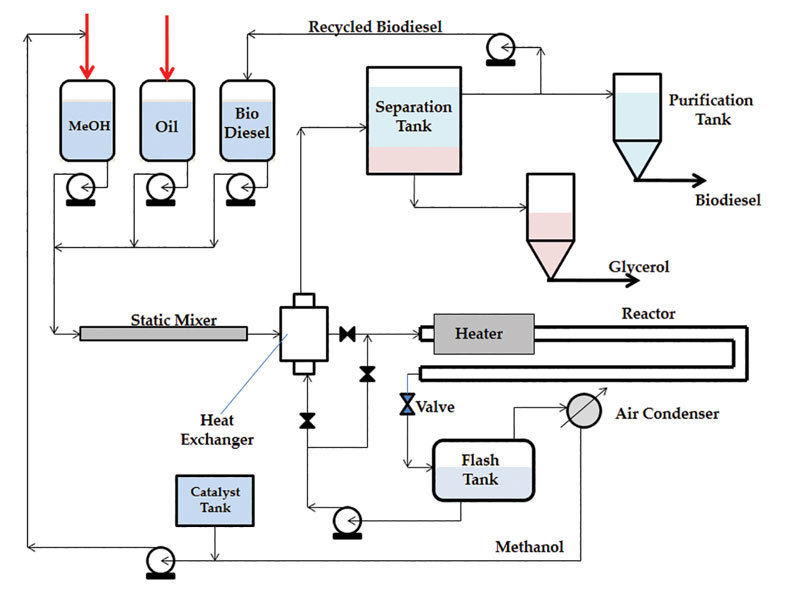

PHOTO: UZI MANN
January 7, 2014
BY Ron Kotrba
Sometimes breakthroughs in biodiesel production technology come in the form of sophisticated processes, complex equipment and migraines from grueling hours spent climbing the learning curve—for both the inventors and the implementers. Other times, a simple, effective problem-solving approach can achieve more promising results. Without question, the suite of process innovations employed at biodiesel plants is, in and of itself, remarkable. From enzymatics to solid acid catalysis, ultrasonic mixing or supercritical processing, the goal of each novel approach is ultimately to lower the cost of production and provide the best possible margins for producers. The fact is, in most cases, biodiesel is manufactured using conventional sodium or potassium hydroxide or methylate catalysts. So, for those producers who are simply looking for ingenious ways to increase production at reduced costs, without gutting their plants and spending millions of dollars on new equipment, systems and engineering expenses, perhaps they should consider an approach described in the recently issued U.S. Patent No. 8,420,841 developed by Drs. Uzi Mann and Stan Emets at Texas Tech University.
The patent is the culmination of more than five years of lab-scale and pilot-plant biodiesel research. Chemical engineering professor Mann has just retired after 35 years at TTU. Emets, Mann’s former postdoctoral researcher at TTU, is now working as a process engineer for Phillips 66. After leaving TTU and prior to joining Phillips 66, he worked at Global Alternative Fuels LLC, a 15 MMgy commercial biodiesel production facility in El Paso, Texas.
“From a very fundamental point of view, if you look at the transesterification reaction, you have two problems that are related to each other,” Mann says. “Problem No. 1 is, you have two reactants, one is the oil or triglycerides and the other is alcohol, which are immiscible under normal conditions. When you try to have a reaction between two immiscible reactants, the reaction takes place only at the interface between the two phases. Because the transesterification reaction is slow in the beginning due to the interfacial limitations, some producers use large reaction vessels with long residence times to carry out the reaction to completion. So, the first problem is how to create a very large interfacial area or, in general, how to eliminate altogether the problem of the interfacial area limitations?”
Problem No. 2, Mann says, is the level of the reactor temperature, which is determined by the boiling temperature of the alcohol. “High reactor temperature is desirable for two reasons,” he says. “First, the transesterification reaction rates are faster, and second, because the reactions are slightly endothermic, their equilibrium compositions contain higher levels of biodiesel. If you want to operate the reactor at high temperatures, a pressurized tank is needed because the reactor pressure should be maintained above the respective boiling point of methanol.” Large, pressurized tanks are inherently expensive, he adds.
The main purpose of the patent is to provide a process for biodiesel production that overcomes these two fundamental problems.
Atomization of Alcohol
Ultrasonics isn’t new to biodiesel; several plants use the technology, vibrating the reactor vessel at high-frequency in order to speed the reaction between the oil or fat and alcohol. Several years ago, Mann’s colleague mentioned the use of ultrasonics in biodiesel production. “With ultrasonic devices vibrating the whole tank, it may affect the reaction in some way, but it’s not addressing the main issue of limiting interfacial area between the two reactants,” Mann says. “Stan and I were fortunate because we were working on another project using ultrasonics—totally different than biodiesel—and then it just occurred to us. We were using ultrasonics to generate very fine droplets of liquid—it was water for that project, but it can apply also to alcohols—and we said, aha, we can make very fine alcohol droplets and mix them into a large volume of oil and, in essence, we are achieving the objective of creating a very large interfacial surface area between the alcohol and the oils or fats.” Thus, the idea was born to use an ultrasonic atomizer to generate ultrafine alcohol microdroplets, and to devise a simple method to disperse them into the oil feed stream using guided inclined plates. Once a dispersion of fine alcohol droplets in oil is created, the rates of the transesterification reactions increase by several orders of magnitude, and a much smaller reactor volume is needed.
Advertisement
Advertisement
Consequently, Mann says, continuous tubular reactors can be employed, which can be readily operated at high temperatures and higher pressures. Figure 1 is a schematic that shows a biodiesel process based on ultrasonic atomization of the alcohol feedstock. The process was demonstrated at TTU on a pilot plant, using a tubular reactor 3 inches in diameter by 20 feet long.
“We developed a continuous process with tubular reactors, and if we want to increase production, we can do so easily in one of two ways,” Mann says. “We can use tubes with a larger diameter, or we can add tubes in parallel. Regardless, we can operate the reactor at higher temperatures, which enable us to recover and recycle most of the alcohol, which hasn’t been converted in the reactor.” A valve is installed at the reactor outlet to control the reactor pressure and maintain it above the corresponding boiling point of the methanol. Mann recommends installing a flash tank at the outlet of the reactor. As the unreacted methanol passes through the valve, it vaporizes out of solution in the flash tank and is recovered in the condenser and recycled back into the process. “I know many producers that either discard the unreacted methanol with the glycerin, and some producers are adding a distillation column to remove the methanol from the glycerin,” he says. “In our process, the reactor temperature is so high (greater than 120 degrees Celsius), relative to the normal boiling point of the methanol, that by using a flash tank and an air condenser, we can recover and recycle most of the methanol.”
Emets adds that by removing the methanol at the outlet of the reactor, the ability to separate the glycerin from biodiesel is substantially enhanced. “That’s an important component of the plant operation,” he says. “Even if methanol is only partially removed, the time needed to separate the glycerin stream from the biodiesel is reduced substantially. When the separation step is the ‘bottleneck,’ this can increase plant production. Some may employ additional purification systems after the separation tank for the biodiesel and glycerol, and I agree with that, but one of the main benefits we’re trying to show in the patent is that you should not ignore the fact that having a flash tank after the reactor enhances the performance downstream.”
“When you compare this atomization approach to other patents using ultrasonics to vibrate the whole reactor, the amount of energy needed to vibrate the reactor is tremendous,” Mann says. “We are interested only in generating very small droplets of methanol in oil. We use the ultrasonic energy just to form droplets of alcohol with high surface area. The size of the droplets can be controlled by the ultrasonic frequency, and the droplet size distribution is very narrow. Cavitation, which occurs in other ultrasonic applications, requires relatively large amount of power.” Emets says, “There’s nothing wrong with achieving cavitation, but the question becomes, what’s the cost of that reactor and the operating expense versus the type of reactor we worked on?” Also, the main drawback of cavitation reactors, they say, is their inability to operate at high temperatures and pressures. “Because,” Mann says, “the bottom line is, in a cavitation reactor, you want to mix up the oil and alcohol, but you don’t want the alcohol to be in the vapor phase.”
Another potential benefit of the process is a smaller amount of excess methanol may be needed because of the higher reactor temperature. In most applications, twice the required theoretical amount of methanol is used in order to push the reversible transesterification reactions in the direction of biodiesel production. If higher equilibrium conversions at high temperatures can be achieved, smaller amount of excess methanol is needed. This is especially important for the competitiveness and economics of large-scale facilities.
Biodiesel as a Cosolvent
In the lab at TTU, Emets observed that, as the reaction proceeds, the dispersion mixture of oils and methanol droplets becomes a clear, homogeneous phase. This implies that a certain amount of biodiesel can serve as a cosolvent for oil and alcohol, forming a homogeneous phase of the mixture. The significance of this observation is that, in the homogeneous phase, the reaction rates are not limited by the interfacial area of the two reactants anymore. Part two of the patent, to be issued shortly, describes a biodiesel process that employs biodiesel as a cosolvent. Figure 2 shows a schematic diagram of this process. A recycle loop carries a slipstream of biodiesel product back to the inlet of the reactor, where it is mixed with the methanol and the oil in the desired proportions.
“Once we don’t have limitations of the interfacial area, the reactions proceed at their intrinsic rates, and we can carry them in a tubular reactor and at a higher temperature and pressure, and push the conversion to the biodiesel direction,” Mann says. “You can look at the biodiesel that is fed into the inlet of the reactor as an inert, a cosolvent, so we are diluting the reactants a bit with the cosolvent.” The penalty for this is a slightly larger reactor. “But the reactor volume is still much smaller than the size of a reactor we would need to react the two immiscible reactants,” Mann explains.
Advertisement
Advertisement
Like many discoveries, the idea of using biodiesel as a cosolvent was stumbled upon in the lab. “We were atomizing ethanol and mixing it with the oil, and we saw that after a short time we got a homogeneous solution,” Mann says. “There was no more two phases.” Again, with a single phase, there were no more limitations by the interfacial area between the two reactants. “We ran some experiments to see how much biodiesel we would need in order to get a homogeneous mixture at room temperature—we did it with methanol and ethanol.”
Emets says, “You can consider it like the limits of making very, very fine droplets of methanol in the oil. When you try to create the smallest droplets possible, you are approaching that condition of biodiesel as a cosolvent. The cosolvent provided the best possible scenario for us, and it gave us the idea that we don’t even have to use ultrasonics to generate alcohol droplets. And when we did that, now the question becomes, what would be the benefits in addition to that?” They found that by adding biodiesel as a cosolvent, they were able to cut the rate of methanol needed for completing the reaction. “In doing some optimizations, we actually created a much simpler setup to get the biodiesel going, and after that, we significantly simplify the process downstream in the purification of biodiesel,” Mann says. Interestingly, Mann’s and Emets’ discovery of using biodiesel as a cosolvent, which is covered in a forthcoming U.S. patent, eliminates the need to implement their original idea—atomizing the methanol. “Adding biodiesel would eliminate the need for ultrasonics or atomization because, in essence, we are forming a homogeneous liquid, so there is no interfacial surface between the reactants anymore,” Mann says. “The reactants are all in one phase, with biodiesel as a cosolvent.”
Mann and Emets cannot explain why biodiesel performs as an effective cosolvent—they are chemical engineers, not chemists. But Mann speculates. “Since biodiesel contains chemical groups from both the oil and the alcohol,” he says, “perhaps it facilitates such that both the alcohol and the oil are miscible in the biodiesel.”
When performing their research at TTU, the benefactor of their work was searching for a solvent that would blend the oil and the methanol into a single phase. “We were looking at if there was a possibility of not introducing any additional chemicals into the process, such that we don’t have to remove it later on,” Emets says. “We can simplify the whole process by not introducing any additional stream other than oil and methanol, resulting in biodiesel, which we can recycle back to the reactor in using it as a cosolvent.”
Next Steps
The patent is owned by TTU, and the university is pursuing commercialization to find a biodiesel producer who’d like to adopt the technology, or technologies, for royalties. “I would like to find a commercial partner to develop the technology on a larger scale and take advantage of it,” Mann says. Ryan Reber, the intellectual property manager at the Office of Technology Commercialization with TTU, tells Biodiesel Magazine, “That’s definitely our first option, trying to find corporate partners and transfer the technology to them. But if that can’t be done, or if they want to see more data or information before they jump in and make a commitment to the technology, we’re looking at talking to some venture investors and entrepreneurs to develop this technology to a point where we can have more information, have more of a proven concept, so we can talk to many of these established companies.”
Emets says even though these approaches are set forth in the published patent now, there are several ways to further improve the technology in the future. “It would be good to show manufacturers where the improvements can be,” he says. “The idea is to concentrate on the reactor itself, which simplifies a lot of other headaches in biodiesel production. It also creates a future for biodiesel technology that addresses other issues like not overdosing the methanol, or reusing as much as possible. Another item that wasn’t discussed in the patent is reduction of the amount of catalyst needed for the reaction to take place, and the negative effects associated with caustic such as production of soaps. After that, utilization of biodiesel as a cosolvent, which is part of the process on a large scale, simplifies the operations of the biodiesel plant.”
Author: Ron Kotrba
Editor, Biodiesel Magazine
218-745-8347
rkotrba@bbiinternational.com
Related Stories
Biodiesel capacity in the U.S. and Canada dipped slightly stable in 2024, with several renewable diesel producers reporting headwinds and lower margins alongside a drove of SAF projects in various stages of development.
The IEA’s Task 39 group has new research regarding the development and status of the sustainable aviation fuel industry.
The U.S. EPA on Nov. 16 released updated RIN data, reporting that nearly 2.11 billion RINs were generated under the RFS in October, up from 1.81 billion generated during the same month of last year.
Conestoga to host SAFFiRE cellulosic ethanol pilot plant
Conestoga Energy and SAFFiRE Renewables LLC announced on Nov. 16 their agreement for Conestoga to host SAFFiRE’s cellulosic ethanol pilot plant at Conestoga’s Arkalon Energy ethanol facility in Liberal, Kansas.
Officials at Calumet Specialty Products Partners L.P. discussed the company’s proposed plans to boost sustainable aviation fuel (SAF) production at its Montana Renewables biorefinery during third quarter earnings call, held Nov. 9.
Upcoming Events










