Searching for effective, environmentally friendly water treatment Technologies

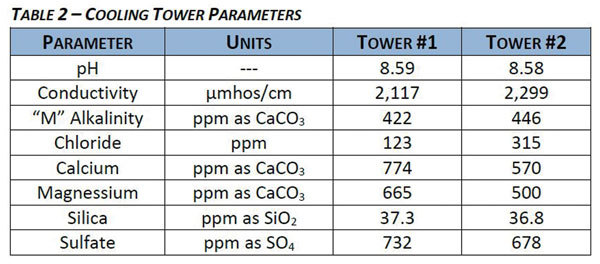
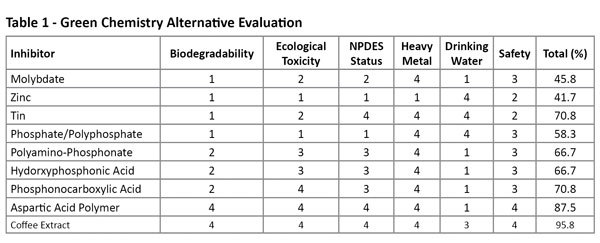


PHOTO: U.S. WATER SERVICES
September 17, 2015
BY Michael Mowbray
There is a heightened focus on environmental sustainability today, and for good reason. Gone are the days of it being acceptable to open valves and discharge unregulated waste streams into the environment. As time has progressed, industry has seen the impact of heightened scrutiny of the public and regulatory bodies. There are strong trends in protecting our natural resources from excessive usage and compromising their quality. Companies, and now even municipalities, are being subject to thorough review and comment periods to justify a facility’s proposed discharge rates and water composition.
Historically, one area that has come up during the environmental review cycle is the evaluation of the additives used to treat cooling tower systems. The plot of the movie “Erin Brockovich” was centered on the use of the cooling tower chemical additive chromate and, when it was discovered that chromates were carcinogenic, the use of chromates was banned.
Today there is a new focus on cooling tower additives. This time, phosphorus (P) discharges have gained significant attention as a result of the impact they can have on the eutrophication of lakes and other waterways. Phosphorus has long been recognized as the controlling factor in plant and algae growth for many lakes and streams. A minor increase in phosphorous can fuel substantial increases in both aquatic plant and algae growth, which can have severe impacts on a community. Phosphorus originates from municipal and industrial water discharges, as well as agricultural runoff. Many parts of the country, including the Great Lakes and Chesapeake Bay watersheds, are regulating the acceptable P discharge levels well below those commonly employed in traditional cooling water treatments for scale and corrosion control. The state of Wisconsin, for example, is regulating P discharges at levels in the range of 0.04 to 0.075 parts per million (ppm). This represents a significant challenge for facilities operating under direct discharge permits.
The use of phosphorus-bearing compounds in industrial cooling water treatment programs has been commonplace since they replaced chromates as corrosion inhibitors in the early 1970s. Typical alkaline all-organic cooling water programs where phosphonates are present as scale inhibitors can have phosphorus levels from 0.3 to 2.5 ppm as P, while stabilized phosphate programs can have phosphorus levels as high as 6.0 to 7.0 ppm. Alternative corrosion inhibitor options may not be viable since they are often based on metals such as zinc or molybdate that are also being closely regulated in terms of acceptable discharge levels. Without an effective corrosion and deposit (scale) control program in place, industrial cooling systems could be compromised in a relatively short time. Facilities could experience significantly higher operating costs as well as potentially impact production capacities, for example, from a loss of vacuum in the surface condensers.
Evaluating Alternatives
Specialty chemical providers have recognized the challenges facing the industry for some time and have been diligently working to find an economically viable solution that can deliver the protection required. Suppliers have investigated nonmetallic corrosion inhibitors and have made them commercially available. These organic inhibitors are often based on low molecular weight polymers, phosphonates and amino phosphonates. Some of these organic molecules have proven to be successful carbon steel corrosion inhibitors. Although they show improved environmental acceptability, they too may have some technical concerns. Organic molecules may be more susceptible to oxidizing biocides than inorganic molecules. Many azole-based copper corrosion inhibitors are susceptible to degradation by oxidizing biocides. Hydroxyphosphonic acid has shown susceptibility to chlorine, even at low levels. This presents a problem because oxidizing biocides are a necessary part of any successful water technology program for control of potentially harmful bacteria, such as legionella. Organic inhibitors based on the phosphorus molecule do not meet the discharge requirement for P due to the reversion of some of the organic phosphonate to orthophosphate. Unfortunately many alternative programs have failed to properly control the corrosion rates in systems without the use of phosphorus-bearing inhibitors. Table 1 illustrates an evaluation of green chemistry alternatives conducted by scientists at St. Michael, Minnesota-based U.S. Water Services Inc. The various alternatives were subjectively rated, with 4 being the best score and 1 the worst.
Informed by their review of the alternatives, scientists at U.S. Water developed new cooling water treatment technology, working with a Midwest ethanol plant to reduce phosphorus concentrations in its discharge. The plant had two different cooling towers due to different process conditions. Both utilized induced draft counterflow cooling towers. The first cooling tower, comprised of mild steel, copper and 304 stainless steel, ran for six months from May to October at an estimated 18,000-gallons-per-minute (GPM) recirculation rate, while the second cooling tower, comprised of only mild steel and 304 stainless steel, ran year round at an estimated 12,600 -GPM recirculation rate.
Traditional water treatment programs were being utilized in the treatment of the cooling towers. The program for Tower No.1 utilized an all-organic program with an azole supplement due to the copper metallurgy contained within the system. Tower No. 2 did not have an azole supplement. The results obtained from the first chemical program were satisfactory, but U.S. Water tried an alternative all-organic program and corrosion rates actually increased slightly from the first program to the second program in Tower No. 2.
Needing a viable chemical treatment alternative due to discharge regulations, the client implemented the new U.S. Water program, trademarked PhosZero. In order to ensure proper system protection, corrosion rates were carefully monitored using corrosion coupons along with a Corrator, a specially designed probe that gives a direct electrochemical measurement of corrosion rate. Corrators are good tools to measure trends and instantaneous relative corrosion rates. Corrosion coupons are an alternative method to monitor the same parameter. Corrosion coupons are samples of pertinent metal (i.e., mild steel or copper) that are preweighed to a high degree of accuracy. These metal samples are put into a system and exposed to the water in the system for an extended duration. After a period of time, the metal samples are removed and sent in for analysis where they are first cleaned of debris and then reweighed. The difference in the metal mass (i.e., metal loss) and the length of exposure (in days) are used to calculate the corrosion rates. In this study, corrosion coupons were employed in addition to the Corrator to monitor the cumulative corrosion rates over a 45-to 60-day exposure period. The results are summarized in Table 3.
The client wanted to maintain the same water efficiency (i.e., not reduce the cycles of concentration) that yielded a Langelier Saturation Index of approximately 2.25. This LSI represents a relatively high scaling potential. The heat transfer surfaces were monitored closely as well, and during the course of the entire trial there was no observed accumulation of scale that would impede the heat transfer efficiencies. Table 2 shows the pertinent cooling tower values during the trial. Corrosion rates improved dramatically within the first five hours of implementation of PhosZero. The corrosion rates for the mild steel, as measured by an online Corrater showed significant improvement during the implementation phase, falling from greater than 2.5 mils per year (mpy) to less than 0.5 mpy. The results were better than anticipated and the primary objective of eliminating phosphorus from the cooling tower chemical treatment program was attained. In addition, the zero phosphorus treatment program provided a significant reduction in the corrosion rates. The corrosion rates dropped from an approximate average of 4 mpy to an average around 1.3 mpy.
The results signify a significant shift in paradigm in the water treatment industry. The norm used to be for facilities to have to settle for compromised results or additional water use when using alternate chemicals that didn’t contain phosphorus. Now, industry is able to meet low P concentrations in their discharges while still protecting their critical assets.
Author: Michael Mowbray
Technical Marketing Manager, U.S. Water Services
763-497-1293
sarah.haug@uswaterservices.com
Contributing Author: Gary Engstrom, Technology Manager, U.S. Water Services
Advertisement
Advertisement
Related Stories
U.S. fuel ethanol capacity fell slightly in April, while biodiesel and renewable diesel capacity held steady, according to data released by the U.S. EIA on June 30. Feedstock consumption was down when compared to the previous month.
XCF Global Inc. on July 8 provided a production update on its flagship New Rise Reno facility, underscoring that the plant has successfully produced SAF, renewable diesel, and renewable naphtha during its initial ramp-up.
FutureFuel idles biodiesel production amidst regulatory uncertainty, shifts full focus to specialty chemicals growth
FutureFuel Corp. on June 17 announced it will temporarily idle its biodiesel facility upon completion of its remaining contractual obligations, anticipated to occur by the end of June. The company is shifting its focus to specialty chemicals.
The U.S. EPA on June 18 announced 1.75 billion RINs were generated under the RFS in May, down from 2.07 billion that were generated during the same period of last year. Total RIN generation for the first five months of 2025 reached 9.06 billion.
TotalEnergies has announced the company expects its facilities will be able to produce more than half a million tons of SAF a year by 2028 to cover the increase in the European SAF blending mandate, set at 6% for 2030.
Upcoming Events










