Bound by Determination

October 13, 2006
BY Ron Kotrba
The demand for scientific research often comes from the need to correct a situation. Companies that produce pharmaceuticals, for instance, are in business because people have ailments for which treatment is desired. Likewise, authorities in the biodiesel industry believe one of the most disconcerting ailments in need of treatment has been the coagulating of fuel in filters and at the bottom of tanks for too long in too many different places, which has spurred the science behind its correction. This research has little to do with biodiesel cold flow issues though. In fact, the circumstances pushing this particular aspect of fuel-quality control are much more elusive than that of cold flow.
The problem is not specific to location, either. Minnesota's statewide clogging of diesel fuel filters last winter after its B2 mandate went into effect exemplifies the problem to a degree. What happened in Washington in the winter of 2004-'05, where similar fuel-related problems occurred in ferry boats fueled by B20 crossing Puget Sound, is a good case in point, too. A lesser-known case out of Utah appears to parallel Minnesota and Washington's symptoms of troubled biodiesel. Frank Anderson of Cardwell Distributing says his company was buying B100 and blending it down to B20 for two months without incident. Once the cooler air hit in October 2005, the blender began to experience filter problems.
"We truck in our B100 from Colorado, and the samples off the trucks are crystal clear," Anderson says. "We put the B100 in a heated, insulated tank. We send our truck to the rack and buy premium diesel with [an additive] package. We then blend 20 percent onto the truck and pump it into an above-ground tank."
Anderson says samples are pulled from the truck after blending-before going into the above-ground tank-and everything still seems fine. After the fuel is pumped into Cardwell's B20 tank, however, Anderson says, "For some reason, the fuel then separates, and there is a fallout of white slime that goes to the bottom of the tank and, in the process, out to our dispenser, [where it] clogs filters there and in our customers' vehicles." He says the bottom of the tank looks like it is covered with a foam that can best be described as "spray insulation." Cardwell Distributing dealt with this issue from October 2005 well into 2006.
Situations of this sort lead to more than just bad press for biodiesel. Individuals in the renewable fuel research community are passionate about biodiesel and its success, and fortunately, they strive to understand the nature of a pervasive problem in order to ultimately fix it.
Dr. Randall von Wedel, principal and director of research with CytoCulture International Inc., tells Biodiesel Magazine that he recently returned from Europe, where he had an informal meeting with top scientists in Berlin to discuss problems with near-ASTM-D-6751 biodiesel fuel in the United States. The information von Wedel obtained from that insightful meeting could explain why near-spec fuel (at or slightly above particular specifications in ASTM) would cause such seemingly aggravated problems.
A Meeting of the Biodiesel Minds
Von Wedel is a biodiesel veteran, who has been researching, testing and using biodiesel since the early 1990s. More recently, he developed a quick B100 field test, called the pHLip Test (see April 2006 Biodiesel Magazine). With his experience, even he doesn't have all the answers behind recent quality problems. "I attended the conclusion of a board of directors meeting for the German Biodiesel Quality Control Board (GBQCB)," von Wedel says. "I visited with three scientists who work for the organization." Among these three scientists was Dr. Jurgen Fischer, senior scientist with the ADM-Connemann Group. "[The GBQCB is] responsible-as we hope to be in the United States-for monitoring and enforcing quality control for biodiesel throughout the country," von Wedel tells Biodiesel Magazine. According to von Wedel, the methodology of the German program includes rigorous sampling, sample collection protocols, sample archives and testing on a regular basis. Von Wedel says while some companies apply similar rigor in the United States, others don't.
"It's actually being done in California because we're gun-shy out here," says von Wedel, who lives and works in the Golden State. "We've had so many instances where we've received railcars [with] a certificate of analysis that says this railcar meets ASTM, but the reality is when we go to look at it, the fuel is turbid, it has precipitates, and if we collect samples off the bottom, it fails." That's what prompted von Wedel's trip to Germany.
Decoding a Convoluted Spec
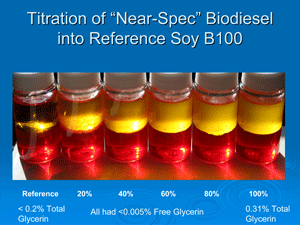
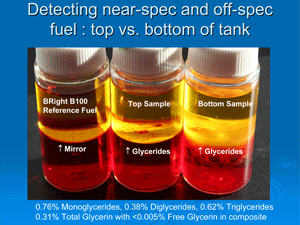
Precipitates have been settling out in biodiesel across the United States. Earlier this year, researchers discovered that some of those instances involved monoglycerides and diglycerides present in the fuel, indicating incomplete transesterification. Free glycerin is rarely a problem, either in the fuel or in meeting the spec. ASTM's total glycerin spec of 0.24 percent is not so widely understood though, von Wedel says. "Total glycerin is an ASTM value that is a calculated value based on measurements," he says. "It consists of four components."
First, there's the free glycerin that is supposed to settle in processing and be removed from the fuel. Simple water-washing accomplishes this. Second, if a producer achieves 96 percent to 97 percent transesterification, there are residuals that don't completely esterify, von Wedel says. "They include, first, the triglyceride, which is really just the unreacted oil or fat," he says, but those are rarely a problem either. "What comes up next are the residuals with the diglycerides, which is the same molecule (as the triglyceride) missing one fatty acid chain," von Wedel says. "You don't want that accumulating in your fuel or your engine." The third one that becomes more problematic is the monoglycerides. Monoglycerides are a problem because they are more prevalent as a contaminant in biodiesel.
"Given X percentage of monoglycerides or Y percentage of monoglycerides, [lab techs] calculate how much of that is the actual glycerin portion," he says, adding that 25 percent of monoglycerides and 12 percent of diglycerides make up the glycerin backbone included in the bound glycerin spec calculations, while the connected long fatty acid chains aren't. "They do the same for the [diglycerides and the triglycerides], and the sum of those three individual numbers represents what is called 'bound glycerin.' They then take the bound glycerin and add it to the free glycerin, which should be very low, and they come up with a total number that's called 'total glycerin free and bound.' That is why you can get 96 [percent] or 97 percent transesterification and still meet the ASTM total glycerin spec of only 0.24 percent. That's the ASTM method. It's awkward, and it's a bit cumbersome. There's nothing particularly wrong with it, but it's deceiving." He says the Germans confirm this test method is still the most reliable test protocol for bound and free glycerin despite its cost and complexity.
Fischer's Hypothesis: Is It a Missing Puzzle Piece?
Monoglycerides, diglycerides and triglycerides are not water-soluble, meaning they don't go away in the wash. If near-complete transesterification hasn't occurred, then the sterols remain dissolved in B100. "If you're under ASTM, meaning you're under the limit of 0.24, you should be OK," von Wedel tells Biodiesel Magazine.
The National Biodiesel Board (NBB) says if one meets ASTM specs and achieves BQ-9000 certification to ensure that those ASTM specs are met, then the rest should fall into place. "The NBB wants to re-emphasize the importance of meeting the ASTM D 6751 spec," says NBB Technical Director Steve Howell. "Using a BQ-9000-approved company provides excellent means to help ensure that only ASTM-grade product is being used."
However, von Wedel says ASTM biodiesel "should" be OK-except when other forces come into play. "There are other molecules, other dark Darth-Vader-type creatures that inhabit the fuel," he says. The mysterious residents in biodiesel that von Wedel refers to are sterols, which are complex aromatic ring structures like cholesterol. "They are found in very low concentrations in all vegetable oils, and they can interact with traces of impurities in biodiesel," he says.
While von Wedel was in Berlin, he learned of Dr. Fischer's hypothesis, which suggests these sterols may aggregate into complexes with monoglycerides and diglycerides present in biodiesel, helping to form a precipitate that falls out of solution. "What it does is amplifies what used to be just a residual trace amount of a [monoglyceride and diglyceride], and they will suddenly form precipitates under certain conditions," von Wedel says.
He adds that moisture in the headspace of railcars shipping B100, combined with shipping time, could explain why B100 might look good leaving the plant but turbid once the biodiesel is ready for unloading. This exposure to "cool, moist air" ought not to be confused with gelling related to cold flow though. After on-spec biodiesel gels at any given temperature, it should be "reanimated" in its entirety once it warms back up. "Gelling is a reversible phenomenon," von Wedel confirms. "We're talking about a precipitate that doesn't go back into solution, which ends up clogging filters as it did in Minnesota, in Washington and, of course, here in California."
Before von Wedel made the trip to Germany, he says he already knew that monoglycerides and diglycerides are hydrophilic. "This does not mean they dissolve in water, but they will interact with water," he says. Monoglycerides and diglycerides are amphoteric, meaning the alcohol "head" of the long chain likes to stay in water, while the "tail" of 18 or so carbon atoms prefer to remain in the fuel, von Wedel tells Biodiesel Magazine.
"I went over all of that with Dr. Fischer," he says. "I said that I thought the precipitates were caused by hydration. He said it could be, but it probably also means that there are traces of sterols and they all slowly form insoluble complexes that precipitate. To me, that was new insight. It was like a new paradigm in my brain."
Today's Approach: Caution Over Detection, Prevention
"I spoke with Randall (von Wedel) about the same issue (of sterols)," says Robert McCormick, principal engineer at the National Renewable Energy Laboratory (NREL). "It's fascinating, and it fits right in with a new project we are going to be starting in the next few months. Hopefully, we will reveal if there is any truth to it."
If Fischer's hypothesis turns out to be true, researchers may give more attention to sterols in biodiesel feedstocks. Proving this hypothesis may be tricky if sterols are hard to detect though. "I asked Dr. Fischer, 'How can we in America test for sterols?'" von Wedel says. Fischer's response translates into, "Sorry, but no," implying that sterols are very hard to measure.
"There is little to no technical information on this," says Dr. Gary Knothe, USDA Agricultural Research Service scientist. "This is an issue now, but work is just beginning. Research will have to show if sterols aid the precipitation of [monoacylglycerols] and diacylglycerols. It could well be that just the presence of these species in greater amounts causes the problem, without one causing the other to precipitate. There may be some other aspects that are yet unknown. Again, we'll have to see what the results are."
Until the information is available, caution looks to be the best practice for producers and blenders. Higher-quality feedstocks seem to help. If a producer uses old restaurant grease as a feedstock, experts instruct the removal of all the impurities for a quality fuel. A more complete transesterification could make fuel less likely to exhibit deposits, too. "If you have correctly performed transesterification, you should not have those [monoglycerides] and diglycerides lingering around to precipitate," von Wedel says.
In the case of Cardwell Distributing's biodiesel issues in Utah, the company says it is still providing biodiesel to its customers. Anderson tells Biodiesel Magazine that Cardwell Distributing's biodiesel supplier has since changed its refining process, and the problems have ceased, at least for now.
Some experts say perhaps the 0.24 spec for total glycerin needs to be lowered to ensure lower total glycerin in the biofuel. Knothe disagrees on certain grounds. "Lowering this specification too much may entail problems for biodiesel lubricity when using blends like B2," he says. "Research has shown that minor components-contaminants-in biodiesel, such as [monoglycerides] and free fatty acids are essential for the lubricity of B2." The NBB's Howell was unavailable for further comment on this matter at press time.
If sterols are difficult to detect directly, there may be an indirect method to test for them. "We know that if they are present, they will fall out of solution when the fuel gets cool and has moisture," von Wedel says. One might suspect that diluting biodiesel with diesel fuel would reduce the B100 problem, but von Wedel says blending down actually amplifies it. Remembering that the alcohol portion of monoglycerides and diglycerides are hydrophilic, he explains why dilution doesn't work. "These materials also remain more soluble in pure biodiesel, and because it's a more oxygen-rich environment, that means it's more polar," he says. "There's no oxygen in diesel fuel. Hydrocarbon molecules are hydrophobic-they don't like water. So what do you expect is going to happen to those molecules?" Monoglycerides and diglycerides are virtually predetermined to fall out of solution even more so in the presence of diesel fuel due to the nature of the molecular chemistry. Fischer's hypothesis is perhaps trace amounts of sterols amplify this phenomenon.
"The very sad thing is that, in the course of unloading biodiesel into diesel fuel to make B2 [in Minnesota], they could have exacerbated the problem," von Wedel tells Biodiesel Magazine. So, in addition to the potential role of sterols present in near-ASTM B100-exposed to moisture, cool temperatures and time-cautionary handling of the fuel could help reduce instances of precipitates settling out.
Washington ferries that used B20 in 2004-'05 ran into issues with precipitates essentially gumming up large centrifuges used onboard to "filter" or isolate contaminants from the fuel before ignition. "They were buying what they thought to be ASTM fuel," von Wedel says. "It turns out that the fuel was left outside, it was put into big tanks that had moisture, and there were cool temperatures. The already-blended B20 was blasted into the hull of the ship at 300 gallons per minute. … Imagine all of the crashing of those molecules. Biodiesel has to be handled gently. Then, of course, it's diluted into diesel fuel, which is more hydrophobic. It was a perfect storm, and in retrospect, it's no wonder they had the problems that they did." Moreover, he says the amount of oxidation occurring through such forceful and overall borderline-negligent handling of the fuel is cause for concern.
Monitoring Oxidation
"I think [the industry] needs to monitor oxidation stability and oxidative degradation," McCormick tells Biodiesel Magazine. Oxidative degradation, he says, is the formation of peroxides-unlimited in ASTM specs-acids, and gums. "This needs to be prevented," McCormick says. "The best way to do this is to make sure that [the] B100 is not exposed to air at high temperatures during processing." McCormick also says including an antioxidant immediately at the point of manufacture before oxidation has a chance to start would be a good practice. "If B100 is of high-enough quality-well below the acid number standard and meeting all other standards easily-it might still go out of spec for acid pretty quickly if it is unstable," he says.
Instability affects the level of precipitates dropping out of the methyl ester solution. "When you oxidize the fuel, you take the double bonds that are in the soybean oil (chains) and you form an epoxide," von Wedel says. "That temporary molecule is unstable, and it will snap. When it snaps, it will either break off entirely to make a carboxylic acid, or the oxygen will reach across and find another molecule that's got a double bond, and you'll make a temporary bridge like a little link between two separate esters." Those initial bridges are the beginning formations of polymers, which also precipitate out of the fuel.
While the presence of precipitates in biodiesel can speak to a number of different causes, including glyceride levels and oxidation, biodiesel fuel within or slightly above ASTM's 0.24 percent allowance for total glycerin free and bound has been determined to be a significant contributing factor of recent quality concerns nationwide. NREL's research, which McCormick says is getting ready to start, and work by others may determine in the coming months whether Fischer's hypothesis on sterols aggravating the nucleation and precipitation of monoglycerides and diglycerides irreversibly out of solution-even percentages within spec-is correct. As Knothe says, we'll have to wait and see.
Ron Kotrba is a Biodiesel Magazine staff writer. Reach him at rkotrba@bbibiofuels.com or (701) 746-8385.
The problem is not specific to location, either. Minnesota's statewide clogging of diesel fuel filters last winter after its B2 mandate went into effect exemplifies the problem to a degree. What happened in Washington in the winter of 2004-'05, where similar fuel-related problems occurred in ferry boats fueled by B20 crossing Puget Sound, is a good case in point, too. A lesser-known case out of Utah appears to parallel Minnesota and Washington's symptoms of troubled biodiesel. Frank Anderson of Cardwell Distributing says his company was buying B100 and blending it down to B20 for two months without incident. Once the cooler air hit in October 2005, the blender began to experience filter problems.
"We truck in our B100 from Colorado, and the samples off the trucks are crystal clear," Anderson says. "We put the B100 in a heated, insulated tank. We send our truck to the rack and buy premium diesel with [an additive] package. We then blend 20 percent onto the truck and pump it into an above-ground tank."
Anderson says samples are pulled from the truck after blending-before going into the above-ground tank-and everything still seems fine. After the fuel is pumped into Cardwell's B20 tank, however, Anderson says, "For some reason, the fuel then separates, and there is a fallout of white slime that goes to the bottom of the tank and, in the process, out to our dispenser, [where it] clogs filters there and in our customers' vehicles." He says the bottom of the tank looks like it is covered with a foam that can best be described as "spray insulation." Cardwell Distributing dealt with this issue from October 2005 well into 2006.
Situations of this sort lead to more than just bad press for biodiesel. Individuals in the renewable fuel research community are passionate about biodiesel and its success, and fortunately, they strive to understand the nature of a pervasive problem in order to ultimately fix it.
Dr. Randall von Wedel, principal and director of research with CytoCulture International Inc., tells Biodiesel Magazine that he recently returned from Europe, where he had an informal meeting with top scientists in Berlin to discuss problems with near-ASTM-D-6751 biodiesel fuel in the United States. The information von Wedel obtained from that insightful meeting could explain why near-spec fuel (at or slightly above particular specifications in ASTM) would cause such seemingly aggravated problems.
A Meeting of the Biodiesel Minds
Von Wedel is a biodiesel veteran, who has been researching, testing and using biodiesel since the early 1990s. More recently, he developed a quick B100 field test, called the pHLip Test (see April 2006 Biodiesel Magazine). With his experience, even he doesn't have all the answers behind recent quality problems. "I attended the conclusion of a board of directors meeting for the German Biodiesel Quality Control Board (GBQCB)," von Wedel says. "I visited with three scientists who work for the organization." Among these three scientists was Dr. Jurgen Fischer, senior scientist with the ADM-Connemann Group. "[The GBQCB is] responsible-as we hope to be in the United States-for monitoring and enforcing quality control for biodiesel throughout the country," von Wedel tells Biodiesel Magazine. According to von Wedel, the methodology of the German program includes rigorous sampling, sample collection protocols, sample archives and testing on a regular basis. Von Wedel says while some companies apply similar rigor in the United States, others don't.
"It's actually being done in California because we're gun-shy out here," says von Wedel, who lives and works in the Golden State. "We've had so many instances where we've received railcars [with] a certificate of analysis that says this railcar meets ASTM, but the reality is when we go to look at it, the fuel is turbid, it has precipitates, and if we collect samples off the bottom, it fails." That's what prompted von Wedel's trip to Germany.
Decoding a Convoluted Spec
Precipitates have been settling out in biodiesel across the United States. Earlier this year, researchers discovered that some of those instances involved monoglycerides and diglycerides present in the fuel, indicating incomplete transesterification. Free glycerin is rarely a problem, either in the fuel or in meeting the spec. ASTM's total glycerin spec of 0.24 percent is not so widely understood though, von Wedel says. "Total glycerin is an ASTM value that is a calculated value based on measurements," he says. "It consists of four components."
First, there's the free glycerin that is supposed to settle in processing and be removed from the fuel. Simple water-washing accomplishes this. Second, if a producer achieves 96 percent to 97 percent transesterification, there are residuals that don't completely esterify, von Wedel says. "They include, first, the triglyceride, which is really just the unreacted oil or fat," he says, but those are rarely a problem either. "What comes up next are the residuals with the diglycerides, which is the same molecule (as the triglyceride) missing one fatty acid chain," von Wedel says. "You don't want that accumulating in your fuel or your engine." The third one that becomes more problematic is the monoglycerides. Monoglycerides are a problem because they are more prevalent as a contaminant in biodiesel.
"Given X percentage of monoglycerides or Y percentage of monoglycerides, [lab techs] calculate how much of that is the actual glycerin portion," he says, adding that 25 percent of monoglycerides and 12 percent of diglycerides make up the glycerin backbone included in the bound glycerin spec calculations, while the connected long fatty acid chains aren't. "They do the same for the [diglycerides and the triglycerides], and the sum of those three individual numbers represents what is called 'bound glycerin.' They then take the bound glycerin and add it to the free glycerin, which should be very low, and they come up with a total number that's called 'total glycerin free and bound.' That is why you can get 96 [percent] or 97 percent transesterification and still meet the ASTM total glycerin spec of only 0.24 percent. That's the ASTM method. It's awkward, and it's a bit cumbersome. There's nothing particularly wrong with it, but it's deceiving." He says the Germans confirm this test method is still the most reliable test protocol for bound and free glycerin despite its cost and complexity.
Fischer's Hypothesis: Is It a Missing Puzzle Piece?
Monoglycerides, diglycerides and triglycerides are not water-soluble, meaning they don't go away in the wash. If near-complete transesterification hasn't occurred, then the sterols remain dissolved in B100. "If you're under ASTM, meaning you're under the limit of 0.24, you should be OK," von Wedel tells Biodiesel Magazine.
The National Biodiesel Board (NBB) says if one meets ASTM specs and achieves BQ-9000 certification to ensure that those ASTM specs are met, then the rest should fall into place. "The NBB wants to re-emphasize the importance of meeting the ASTM D 6751 spec," says NBB Technical Director Steve Howell. "Using a BQ-9000-approved company provides excellent means to help ensure that only ASTM-grade product is being used."
However, von Wedel says ASTM biodiesel "should" be OK-except when other forces come into play. "There are other molecules, other dark Darth-Vader-type creatures that inhabit the fuel," he says. The mysterious residents in biodiesel that von Wedel refers to are sterols, which are complex aromatic ring structures like cholesterol. "They are found in very low concentrations in all vegetable oils, and they can interact with traces of impurities in biodiesel," he says.
While von Wedel was in Berlin, he learned of Dr. Fischer's hypothesis, which suggests these sterols may aggregate into complexes with monoglycerides and diglycerides present in biodiesel, helping to form a precipitate that falls out of solution. "What it does is amplifies what used to be just a residual trace amount of a [monoglyceride and diglyceride], and they will suddenly form precipitates under certain conditions," von Wedel says.
He adds that moisture in the headspace of railcars shipping B100, combined with shipping time, could explain why B100 might look good leaving the plant but turbid once the biodiesel is ready for unloading. This exposure to "cool, moist air" ought not to be confused with gelling related to cold flow though. After on-spec biodiesel gels at any given temperature, it should be "reanimated" in its entirety once it warms back up. "Gelling is a reversible phenomenon," von Wedel confirms. "We're talking about a precipitate that doesn't go back into solution, which ends up clogging filters as it did in Minnesota, in Washington and, of course, here in California."
Before von Wedel made the trip to Germany, he says he already knew that monoglycerides and diglycerides are hydrophilic. "This does not mean they dissolve in water, but they will interact with water," he says. Monoglycerides and diglycerides are amphoteric, meaning the alcohol "head" of the long chain likes to stay in water, while the "tail" of 18 or so carbon atoms prefer to remain in the fuel, von Wedel tells Biodiesel Magazine.
"I went over all of that with Dr. Fischer," he says. "I said that I thought the precipitates were caused by hydration. He said it could be, but it probably also means that there are traces of sterols and they all slowly form insoluble complexes that precipitate. To me, that was new insight. It was like a new paradigm in my brain."
Today's Approach: Caution Over Detection, Prevention
"I spoke with Randall (von Wedel) about the same issue (of sterols)," says Robert McCormick, principal engineer at the National Renewable Energy Laboratory (NREL). "It's fascinating, and it fits right in with a new project we are going to be starting in the next few months. Hopefully, we will reveal if there is any truth to it."
If Fischer's hypothesis turns out to be true, researchers may give more attention to sterols in biodiesel feedstocks. Proving this hypothesis may be tricky if sterols are hard to detect though. "I asked Dr. Fischer, 'How can we in America test for sterols?'" von Wedel says. Fischer's response translates into, "Sorry, but no," implying that sterols are very hard to measure.
"There is little to no technical information on this," says Dr. Gary Knothe, USDA Agricultural Research Service scientist. "This is an issue now, but work is just beginning. Research will have to show if sterols aid the precipitation of [monoacylglycerols] and diacylglycerols. It could well be that just the presence of these species in greater amounts causes the problem, without one causing the other to precipitate. There may be some other aspects that are yet unknown. Again, we'll have to see what the results are."
Until the information is available, caution looks to be the best practice for producers and blenders. Higher-quality feedstocks seem to help. If a producer uses old restaurant grease as a feedstock, experts instruct the removal of all the impurities for a quality fuel. A more complete transesterification could make fuel less likely to exhibit deposits, too. "If you have correctly performed transesterification, you should not have those [monoglycerides] and diglycerides lingering around to precipitate," von Wedel says.
In the case of Cardwell Distributing's biodiesel issues in Utah, the company says it is still providing biodiesel to its customers. Anderson tells Biodiesel Magazine that Cardwell Distributing's biodiesel supplier has since changed its refining process, and the problems have ceased, at least for now.
Some experts say perhaps the 0.24 spec for total glycerin needs to be lowered to ensure lower total glycerin in the biofuel. Knothe disagrees on certain grounds. "Lowering this specification too much may entail problems for biodiesel lubricity when using blends like B2," he says. "Research has shown that minor components-contaminants-in biodiesel, such as [monoglycerides] and free fatty acids are essential for the lubricity of B2." The NBB's Howell was unavailable for further comment on this matter at press time.
If sterols are difficult to detect directly, there may be an indirect method to test for them. "We know that if they are present, they will fall out of solution when the fuel gets cool and has moisture," von Wedel says. One might suspect that diluting biodiesel with diesel fuel would reduce the B100 problem, but von Wedel says blending down actually amplifies it. Remembering that the alcohol portion of monoglycerides and diglycerides are hydrophilic, he explains why dilution doesn't work. "These materials also remain more soluble in pure biodiesel, and because it's a more oxygen-rich environment, that means it's more polar," he says. "There's no oxygen in diesel fuel. Hydrocarbon molecules are hydrophobic-they don't like water. So what do you expect is going to happen to those molecules?" Monoglycerides and diglycerides are virtually predetermined to fall out of solution even more so in the presence of diesel fuel due to the nature of the molecular chemistry. Fischer's hypothesis is perhaps trace amounts of sterols amplify this phenomenon.
"The very sad thing is that, in the course of unloading biodiesel into diesel fuel to make B2 [in Minnesota], they could have exacerbated the problem," von Wedel tells Biodiesel Magazine. So, in addition to the potential role of sterols present in near-ASTM B100-exposed to moisture, cool temperatures and time-cautionary handling of the fuel could help reduce instances of precipitates settling out.
Washington ferries that used B20 in 2004-'05 ran into issues with precipitates essentially gumming up large centrifuges used onboard to "filter" or isolate contaminants from the fuel before ignition. "They were buying what they thought to be ASTM fuel," von Wedel says. "It turns out that the fuel was left outside, it was put into big tanks that had moisture, and there were cool temperatures. The already-blended B20 was blasted into the hull of the ship at 300 gallons per minute. … Imagine all of the crashing of those molecules. Biodiesel has to be handled gently. Then, of course, it's diluted into diesel fuel, which is more hydrophobic. It was a perfect storm, and in retrospect, it's no wonder they had the problems that they did." Moreover, he says the amount of oxidation occurring through such forceful and overall borderline-negligent handling of the fuel is cause for concern.
Monitoring Oxidation
"I think [the industry] needs to monitor oxidation stability and oxidative degradation," McCormick tells Biodiesel Magazine. Oxidative degradation, he says, is the formation of peroxides-unlimited in ASTM specs-acids, and gums. "This needs to be prevented," McCormick says. "The best way to do this is to make sure that [the] B100 is not exposed to air at high temperatures during processing." McCormick also says including an antioxidant immediately at the point of manufacture before oxidation has a chance to start would be a good practice. "If B100 is of high-enough quality-well below the acid number standard and meeting all other standards easily-it might still go out of spec for acid pretty quickly if it is unstable," he says.
Instability affects the level of precipitates dropping out of the methyl ester solution. "When you oxidize the fuel, you take the double bonds that are in the soybean oil (chains) and you form an epoxide," von Wedel says. "That temporary molecule is unstable, and it will snap. When it snaps, it will either break off entirely to make a carboxylic acid, or the oxygen will reach across and find another molecule that's got a double bond, and you'll make a temporary bridge like a little link between two separate esters." Those initial bridges are the beginning formations of polymers, which also precipitate out of the fuel.
While the presence of precipitates in biodiesel can speak to a number of different causes, including glyceride levels and oxidation, biodiesel fuel within or slightly above ASTM's 0.24 percent allowance for total glycerin free and bound has been determined to be a significant contributing factor of recent quality concerns nationwide. NREL's research, which McCormick says is getting ready to start, and work by others may determine in the coming months whether Fischer's hypothesis on sterols aggravating the nucleation and precipitation of monoglycerides and diglycerides irreversibly out of solution-even percentages within spec-is correct. As Knothe says, we'll have to wait and see.
Ron Kotrba is a Biodiesel Magazine staff writer. Reach him at rkotrba@bbibiofuels.com or (701) 746-8385.
Advertisement
Advertisement
Upcoming Events





