Coming Soon: New Processes





December 20, 2010
BY Luke Geiver
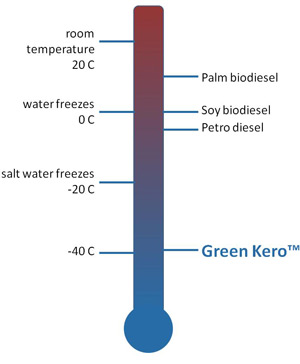
Chad Joshi wanted to eliminate the presence of oxygen within the biodiesel mix. To do it, he looked for people who had knowledge of biofuel chemistry. The CEO of Altranex, a startup company determined to produce a fuel that works in cold weather (as in minus 30 degrees Celsius), Joshi says he wanted people who "understood what needed to be done." The people he eventually found, Jason Sello and Aaron Socha at Brown University, were chosen more on their ability and "understanding" of biofuel chemistry than by any proven cold flow work. But, if the research team's track record predicts success, then Altranex may be producing a biodiesel mix of the future-what Joshi calls "Green Kero"-that performs in cold climates and, very likely, does it without the presence of oxygen.
Rachel Burton, the research and analytical director of Piedmont Biofuels, doesn't have much work interest in oxygen, but she, like the team from Altranex, is part of an innovative research and process design effort that may someday change the face of the biodiesel industry-and she will do it with enzymes. After meeting a member of Novozymes at a 2008 speaking engagement, Burton formed a relationship that would eventually combine her skills with the sustainable biodiesel team of the Denmark enzyme giant. Now, whether its oxygen elimination or wastewater reduction via enzymatic processing, there is no shortage of novel process method research efforts, all of which are tackling the challenges of chemistry. The methods may not be commercially here today, and they may not be quite here tomorrow either, but work such as Burton's and Joshi's show that new approaches are bubbling towards the surface.
The History of Green Kero
To say the creation of the Altranex team and its quest for a cold-climate, biodiesel-like fuel started with the easiest of revelations-that in northern regions the air is anything but warm much of the year and certain biodiesel blends begin to gel in these conditions-would only be half the story. Joshi, who says his oxygen-devoid "Green Kero" could act as either a fuel additive to help lower the cloud point of biodiesel, or eventually even as a fuel itself, points out that work by Sello and Socha on waste vegetable oil process techniques also piqued his interest, helping bring the team together.
The work, which is based on converting triacylglcerols and fatty acids found in waste vegetable oils, implements a single catalyst vessel combined with a microwave reactor. Using Lewis acidic metal catalysts scandium triflate and bismuth triflate in a single reaction to create methyl esters, the two found a way to reduce the reactions needed from two to one. With the help of the microwave reactor to induce temperatures reaching 150 degrees Celsius, the team from Brown was able to produce a reaction in roughly 20 minutes. The metal catalysts could be reused up to five times at 97 percent efficiency, and although the energy required to run the microwave reactor was greater than typical energy requirements, Socha says, the overall energy usage is less given the shorter amount of time needed for the reactions.
Although the WVO process method was only performed at bench scale, and Sello and Socha haven't tested any low-grade grease at this point, small-scale chemistry achievements can sometimes induce big plans (e.g., Altranex). "The stuff they (Sello and Socha) are doing over at Brown really dovetails with some of the processing we want to do to produce the Green Kero," Joshi says. The same catalyst process used in the WVO project would help in one of the steps of the Altranex process. One of the advantages of the solid catalyst system developed by the Brown team, according to Joshi, is that unlike conventional potassium hydroxide-based catalysts that must be removed, the Brown version will cut down on the removal expense due to the recyclability of the catalyst. And it doesn't hurt that the team from Brown knows a thing or two about chemistry.
"We've been thinking about these issues for quite a while," Sello says about his team's work with biodiesel conversion and the chemistry approaches that could make it more efficient. Now, Sello, Socha and others will begin tackling the problem posed by Joshi, making a fuel unphased by cold temps. "The problem with biodiesel," Joshi says, "is that is has some oxygen in it. And the oxygen in some sense is responsible for the high cloud point." Oxygen is at one end of the molecule, Joshi explains, and the methyl esters are at the other end of the molecule. The oxygen present on the molecules tends to attract hydrogen from adjacent molecules due to something known as the Van der Waals force, a weak force that pulls hydrogen towards other molecules. "That attraction is what causes the high gel point," Joshi says. If you get rid of oxygen, that attraction isn't there anymore and the cloud point will drop, and the result will be a fuel similar to Green Kero developed by Sello and Socha.
"We expect that we will be able to make small quantities of Green Kero by this time next year," Joshi says. "Instead of doing a B2 or B5 blend, we could enable B20 to B50 blends that could be run in the winter." The process will utilize multiple feedstocks, including one familiar to the Brown team, waste vegetable oil. Although Joshi is hesitant to discuss the process method further due to patent applications still pending, he says there is already interest in his product. "We've talked with people at Irving Oil, Global Partners and Mansfield Oil," he says, "and they all see the value of what is we are doing. According to Joshi, Global Partners, which has a facility in Albany, N.Y., has shown interest in trying the product as a winter blending agent. "They have a demand for about 25 MMgy of kerosene that we could substitute for," he says.
Sello says he working on multiple biofuels projects at the moment, including the continuation of the microwave reactor metal catalyst method along with the new work for Altranex. "There is a movement at Brown to create a greater presence in the energy area," Sello says, "and there has been a lot of discussion about energy."
Sustainable Energy via Enzymes
Part of the Altranex approach is based on the ability of a fuel additive product to be sustainable, and Rachel Burton is no stranger to that concept. "Piedmont Biofuels approach has always looked to be leading in biodiesel sustainability," she explains. Add the help of Novozymes, and the concept of sustainability that Piedmont Biofuels strives for has bred a new free fatty acid pretreatment method that Burton says has already been proven (at a small scale).
Burton says as a biodiesel producer operating under current market conditions, being able to process lower-quality feedstock high in free fatty acids is what several producers gravitate towards, but doing it without creating a significant amount of waste is the approach Piedmont is taking. "A number of technologies do exist to process the high free fatty acid, low-quality fats and oils but they do create either more waste water or more acidic methanol streams, or they use excessive amounts of methanol," she says.
The enzymatic approach provides a significant reduction in the amount of wastewater, Burton says. "When you use enzymes as opposed to sodium or potassium hydroxide for basic reactions," she says, "you are not going to create soap. By just having the soap in the process, you are creating a significant amount of wastewater because you have to use water to wash out the soap." Along with a soap-free reaction, the coproduct is higher quality. "The coproduct doesn't have any of the soaps or impurities," Burton says. This, in turn, creates a higher value coproduct.
And if you don't believe Burton's assertions that reducing the amount of wastewater used in the biodiesel production process is important, look at part of the funding awarded to Piedmont. Not surprisingly, the team received a U.S. DOE grant for waste water reduction, and it is in the second phase of the grant, worth $1 million over two years.
The team is mainly focusing on Novozymes' 435 enzyme, the most commonly used lipase in biocatalysis. It can be used in both batch and column reaction operations and is also used in industrial processes as a catalyst in the synthesis of simple esters (including polyesters), according to Novozymes. "We've been working on this for at least a year and a half," Burton says, "so we are fairly new to the enzyme biodiesel research body. We approach it as, can we make this process meet the commercial specifications? We've shown some success in being able to have a process that meets the ASTM specification."
The Piedmont process won't be commercially available tomorrow, but in two years, Burton says they hope to have a scaled-up version. Between now and then, however, the work will continue, and it is safe to say that even if the reason for innovation may differ from one research team to the next, as Altranex and Piedmont show, the challenge of chemistry will never be enough to stop novel process method ideas from boiling over into mainstream use.
Author: Luke Geiver
Associate Editor, Biodiesel Magazine
(701) 738-4944
lgeiver@bbiinternational.com
Rachel Burton, the research and analytical director of Piedmont Biofuels, doesn't have much work interest in oxygen, but she, like the team from Altranex, is part of an innovative research and process design effort that may someday change the face of the biodiesel industry-and she will do it with enzymes. After meeting a member of Novozymes at a 2008 speaking engagement, Burton formed a relationship that would eventually combine her skills with the sustainable biodiesel team of the Denmark enzyme giant. Now, whether its oxygen elimination or wastewater reduction via enzymatic processing, there is no shortage of novel process method research efforts, all of which are tackling the challenges of chemistry. The methods may not be commercially here today, and they may not be quite here tomorrow either, but work such as Burton's and Joshi's show that new approaches are bubbling towards the surface.
The History of Green Kero
To say the creation of the Altranex team and its quest for a cold-climate, biodiesel-like fuel started with the easiest of revelations-that in northern regions the air is anything but warm much of the year and certain biodiesel blends begin to gel in these conditions-would only be half the story. Joshi, who says his oxygen-devoid "Green Kero" could act as either a fuel additive to help lower the cloud point of biodiesel, or eventually even as a fuel itself, points out that work by Sello and Socha on waste vegetable oil process techniques also piqued his interest, helping bring the team together.
The work, which is based on converting triacylglcerols and fatty acids found in waste vegetable oils, implements a single catalyst vessel combined with a microwave reactor. Using Lewis acidic metal catalysts scandium triflate and bismuth triflate in a single reaction to create methyl esters, the two found a way to reduce the reactions needed from two to one. With the help of the microwave reactor to induce temperatures reaching 150 degrees Celsius, the team from Brown was able to produce a reaction in roughly 20 minutes. The metal catalysts could be reused up to five times at 97 percent efficiency, and although the energy required to run the microwave reactor was greater than typical energy requirements, Socha says, the overall energy usage is less given the shorter amount of time needed for the reactions.
Although the WVO process method was only performed at bench scale, and Sello and Socha haven't tested any low-grade grease at this point, small-scale chemistry achievements can sometimes induce big plans (e.g., Altranex). "The stuff they (Sello and Socha) are doing over at Brown really dovetails with some of the processing we want to do to produce the Green Kero," Joshi says. The same catalyst process used in the WVO project would help in one of the steps of the Altranex process. One of the advantages of the solid catalyst system developed by the Brown team, according to Joshi, is that unlike conventional potassium hydroxide-based catalysts that must be removed, the Brown version will cut down on the removal expense due to the recyclability of the catalyst. And it doesn't hurt that the team from Brown knows a thing or two about chemistry.
"We've been thinking about these issues for quite a while," Sello says about his team's work with biodiesel conversion and the chemistry approaches that could make it more efficient. Now, Sello, Socha and others will begin tackling the problem posed by Joshi, making a fuel unphased by cold temps. "The problem with biodiesel," Joshi says, "is that is has some oxygen in it. And the oxygen in some sense is responsible for the high cloud point." Oxygen is at one end of the molecule, Joshi explains, and the methyl esters are at the other end of the molecule. The oxygen present on the molecules tends to attract hydrogen from adjacent molecules due to something known as the Van der Waals force, a weak force that pulls hydrogen towards other molecules. "That attraction is what causes the high gel point," Joshi says. If you get rid of oxygen, that attraction isn't there anymore and the cloud point will drop, and the result will be a fuel similar to Green Kero developed by Sello and Socha.
"We expect that we will be able to make small quantities of Green Kero by this time next year," Joshi says. "Instead of doing a B2 or B5 blend, we could enable B20 to B50 blends that could be run in the winter." The process will utilize multiple feedstocks, including one familiar to the Brown team, waste vegetable oil. Although Joshi is hesitant to discuss the process method further due to patent applications still pending, he says there is already interest in his product. "We've talked with people at Irving Oil, Global Partners and Mansfield Oil," he says, "and they all see the value of what is we are doing. According to Joshi, Global Partners, which has a facility in Albany, N.Y., has shown interest in trying the product as a winter blending agent. "They have a demand for about 25 MMgy of kerosene that we could substitute for," he says.
Sello says he working on multiple biofuels projects at the moment, including the continuation of the microwave reactor metal catalyst method along with the new work for Altranex. "There is a movement at Brown to create a greater presence in the energy area," Sello says, "and there has been a lot of discussion about energy."
Sustainable Energy via Enzymes
Part of the Altranex approach is based on the ability of a fuel additive product to be sustainable, and Rachel Burton is no stranger to that concept. "Piedmont Biofuels approach has always looked to be leading in biodiesel sustainability," she explains. Add the help of Novozymes, and the concept of sustainability that Piedmont Biofuels strives for has bred a new free fatty acid pretreatment method that Burton says has already been proven (at a small scale).
Burton says as a biodiesel producer operating under current market conditions, being able to process lower-quality feedstock high in free fatty acids is what several producers gravitate towards, but doing it without creating a significant amount of waste is the approach Piedmont is taking. "A number of technologies do exist to process the high free fatty acid, low-quality fats and oils but they do create either more waste water or more acidic methanol streams, or they use excessive amounts of methanol," she says.
The enzymatic approach provides a significant reduction in the amount of wastewater, Burton says. "When you use enzymes as opposed to sodium or potassium hydroxide for basic reactions," she says, "you are not going to create soap. By just having the soap in the process, you are creating a significant amount of wastewater because you have to use water to wash out the soap." Along with a soap-free reaction, the coproduct is higher quality. "The coproduct doesn't have any of the soaps or impurities," Burton says. This, in turn, creates a higher value coproduct.
And if you don't believe Burton's assertions that reducing the amount of wastewater used in the biodiesel production process is important, look at part of the funding awarded to Piedmont. Not surprisingly, the team received a U.S. DOE grant for waste water reduction, and it is in the second phase of the grant, worth $1 million over two years.
The team is mainly focusing on Novozymes' 435 enzyme, the most commonly used lipase in biocatalysis. It can be used in both batch and column reaction operations and is also used in industrial processes as a catalyst in the synthesis of simple esters (including polyesters), according to Novozymes. "We've been working on this for at least a year and a half," Burton says, "so we are fairly new to the enzyme biodiesel research body. We approach it as, can we make this process meet the commercial specifications? We've shown some success in being able to have a process that meets the ASTM specification."
The Piedmont process won't be commercially available tomorrow, but in two years, Burton says they hope to have a scaled-up version. Between now and then, however, the work will continue, and it is safe to say that even if the reason for innovation may differ from one research team to the next, as Altranex and Piedmont show, the challenge of chemistry will never be enough to stop novel process method ideas from boiling over into mainstream use.
Author: Luke Geiver
Associate Editor, Biodiesel Magazine
(701) 738-4944
lgeiver@bbiinternational.com
Advertisement
Advertisement
Upcoming Events





