Controlling Bacteria During Corn Mash Fermentation




June 10, 2011
BY Reed Semenza
In ethanol production, yeast converts sugar into ethanol. Other micro-organisms, however, including lactic acid and acetic acid bacteria, and others, can contaminate the process. When acid bacteria grow, they compete for the supply of sugar with yeast, resulting in less sugar for ethanol production. Also acid-forming bacteria can create low pH conditions that tend to inhibit the growth of the ethanol-producing yeast.
In order to control the growth of acid producing bacteria, many ethanol plants add antibiotics to the fermentation tanks. The antibiotics allow the yeast to kill much of the acid bacteria but the antibiotics apparently do not harm the yeast. The current method calls for 3 to 5 pounds of antibiotic, usually Virginiamycin, per 500,000 gallons of corn mash in the fermenter. The actual dose of antibiotics is determined by the level of lactic acid in the corn mash during the first 30 hours of fermentation.
The main disadvantage of antibiotics is that they carry through the fermentation process and distillation process and end up in the distillers grains. The DGs provide a valuable feed product but with trace antibiotics, many cattle feeders are reluctant to use DGs or must ration the DGs in the animal feed. Trace antibiotics in the DGs are thought to cause bacteria in cows to mutate to an antibiotic-resistant strain. The U.S. Food and Drug Administration is currently considering banning the use of antibiotics in ethanol production due to the carryover of trace amounts of antibiotics.
A second disadvantage of antibiotics is that acid bacteria can become resistant over time, rendering the use of antibiotics less effective and resulting in ethanol production losses.
DeLaval conducted trials examining the elimination of antibiotics through the use of a blend of oxidizers added to the fermenter—the trademarked organic oxidizer DeLasan MP, a 15 percent peracetic acid (PAA) distributed by DeLaval, plus the inorganic oxidizer, hydrogen peroxide (HP). The primary objective of the test was to determine the most economical dose of Delasan MP and to determine the relationship between lactic and acetic bacteria reduction and dose. Secondary objectives included measurement of the final fermentor ethanol percentage as well as the DeLasan MP decay rates. Decay rate is measured in order to determine residual levels of PAA and HP in the DGs.
The trials were conducted at a 20 MMgy plant in Torrington Wyo., a modified Delta T design. The management wanted to reduce or eliminate the use of antibiotics in an effort to produce antibiotic-free distillers grains with a second objective of increasing ethanol yield.
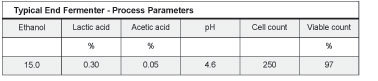
Process Parameters
The process flow for the fermenter begins when the corn mash enters the Stage 1 cooler at 180 degrees Fahrenheidt and is cooled to 140 F. It then enters the Stage 2 cooler and is cooled to 90 F. Flow through the cooler is 110 gallons per minute (gpm). From the stage two cooler, the mash is sent to the fermenter where it is recirculated through a plate and frame mash cooler. When the fermenter has been filled for 30 minutes, yeast is added to the recirculating corn mash. The fermenter takes about 23 hours to fill. Once filled, fermentation begins to accelerate, releasing heat, which is dissipated in the fermenter cooler. The fermenter mash is sent to distillation about 56 hours after the fermenter is filled. The 700-gallon mash cooler has a recirculating rate of 110 gpm, a pH range of 5.5 to 6.5 and in/out temperatures of 180 and 90 F. The 185,000 gallon fermenter has a recirculation rate of 400 gpm, a pH range of 4 to 5.5 and in/out temperatures of 95 and 90 F.
During fermentation, carbohydrates, ethanol and organic acids are monitored in order to insure that the fermentation process is occurring normally and to insure that undesirable bacteria are kept under control. Also, the mash temperature is kept in a range of 90 to 95 F. Typical ranges for pH and organic acids are as follows:
• pH starts at 6.0 then slowly drops to 4.2 after 18 hours. Toward the end of fermentation, pH rises to 4.6.
• Lactic acid percent starts at 0.1 and stays mostly below 0.4 percent when lactic acid bacteria are under control when lactic acid bacteria are present in larger numbers, the lactic acid percentage can rise to as high as 0.8.
• Acetic acid generally starts in a range of 0.03 to 0.05 percent and rises to 0.1. A rise above 0.15 percent results in reduced final ethanol and indicates that bacteria counts are out of control.
The Test
In the trial, DeLasan MP and 31 percent hydrogen peroxide (HP) were added at a rate of 0.8 and 1 gallon per hour, respectively, through a stainless steel pipe carrying the mixture to a header located between the first- and second-stage mash cooler. At the point of injection, the mash temperature was 140 F and the mash flow rate was 110 gpm. The pumps used to pump both the HP and DeLasan MP were Prominent diaphragm pumps fitted with Teflon liquid ends. The dose of the Delasan MP was 15 ppm (as PAA) and the dose of the HP was 40 ppm (as peroxide). However, the two oxidizers were fed for only 15 hours of the 23 hour fermentor fill time, making the overall dose 9.8 ppm of Delasan MP and 26 ppm of HP.
Samples were taken every hour at the mash cooler exit and fermentation tank and tested using a Hach DPD total chlorine test. One DPD powder pillow was added to a 3 milliliter sample in a test tube, stirred gently for 5 seconds and a pink color was observed at the bottom of the test tube. PAA concentration was estimated based on the hue of pink color after 30 seconds. HP concentration was estimated after six minutes. Normal corn mash tests on carbohydrates, ethanol, and organic acids were taken by plant personnel every six hours. Results were compared with normal historical averages when antibiotics were used.
Four months of test results indicated the following:
• In the mash cooler, PAA concentrations consistently measured at 3 to 4 ppm. HP was always positive with an estimated concentration of 10 to 15 ppm.
• In the fermenter, the corn mash was positive for PAA up to two hours into filling. After two hours, PAA was not detectable. HP was not detectable after three hours.
• Both lactic and acetic acids stayed within normal ranges. When both DeLasan MP and antibiotics were used, lactic acid and especially acetic acid levels were lower than when only antibiotics were used. Lactic acid generally ranged from 0.2 to 0.5 percent. Acetic acid generally ranged from 0.05 to 0.12 percent.
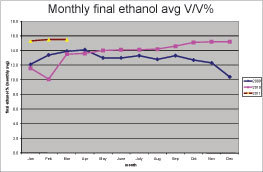
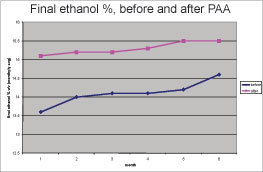
• Alcohol production was 8.5 percent higher than the previous six months (see Charts 1 and 2), even when organic acids did not decrease from normal. This result was not expected as alcohol production is thought to be inversely correlated with organic acids. Higher organic acid concentration usually results in lower ethanol concentration. It is likely that the removal of biofilms on transfer, heat exchanger and tank surfaces resulted in decreased glucose losses and that the extra glucose was converted into ethanol.
The overall test results were extremely positive, indicating that the DeLasan program can effectively replace the use of antibiotics in preventing ethanol loss due to the formation of organic acids. No negative effects of the program were noted based on the balance of carbohydrates, ethanol and organic acids taken in all three fermenters. Also, yeast cell counts and viability tests were all in the normal range.
In addition to helping the plant achieve antibiotic free DGs, the DeLasan program met the following objectives:
• Because the DeLasan is effective at very low dose, the program cost was less than the cost of the antibiotics plus Fermasure.
• The DeLasan program resulted in a net increase in ethanol production of 8.5 percent, which in a 20 MMgy plant amounts to increased profits of $3.5 million per year.
• The DeLasan is added directly from the totes into the corn mash pipe. No mixing, other than the natural turbulence in the pipe, is required. The control test is a modified total chlorine test and is taken at the mash cooler discharge.
• Unlike antibiotics and sodium chlorite, DeLasan does not contribute antibiotic residue or inorganic salts to the DGs or backset.
Author: Reed Semenza
Business and Technical Manager, DeLaval
(209) 996-3657
Reed.Semenza@Delaval.com
Advertisement
Advertisement
Advertisement
Advertisement
Upcoming Events





