Keep water content in methanol to a minimum
June 15, 2010
BY Raj Mosali, president, Jatrodiesel
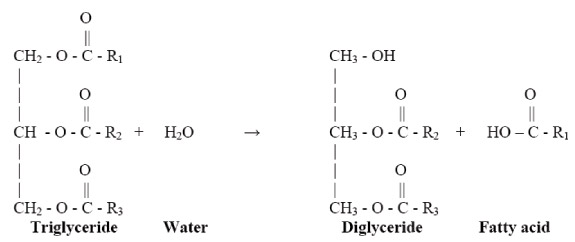
In the production of biodiesel from either high free fatty acid (FFA) or a low FFA feedstock, water in methanol is a huge problem. When water is present, particularly at high temperatures, it can hydrolyze the triglycerides to diglycerides and form FFAs.
When water is present in the reaction, it generally manifests itself through excessive soap production. The soaps of saturated fatty acids tend to solidify at ambient temperatures, so a reaction mixture with excessive soap may gel and form a semi-solid mass that is very difficult to recover.
Some water in the system can be tolerated, because R-O- is a stronger base than OH-, so the transesterification reaction occurs at a higher rate than the saponification of glycerol, leading to FFAs. As a result of the slow reaction rate, only very minor amounts of FFAs are formed during transesterification if the reaction is free of water at the beginning.
Proceeding with transesterification with a water level in methanol higher than 500 parts per million not only leads to an undesirable byproduct of FFA, but also makes the fuel hard to pass the ASTM specifications for water and sediments, acid number and oxidation stability.
Prior to the process reactions, the obvious source of water is the feedstock. Care should be taken to make sure the feedstock has as little water possible, since no one wants to pay for water and then use precious energy drying the oil to get rid of it. Also, the premixed liquid methanol and catalyst bought should be checked for water content to make sure it has a negligible amount of moisture. Prior to the delivery, if the oil, methanol and the catalyst from vendors stay out in the open for very long, they tend to absorb water, so any quality checks have to be performed before unloading the material.
During the reactions, the major source of water in the transesterification reaction is the water carryover in the impure methanol from esterification and prior transesterification reactions (if the methanol is not purified).
Drying biodiesel and glycerin off of methanol is critical for meeting ASTM specs and to transport glycerin. Due to this drying process, used or wet methanol will consist of water with high biological oxygen demand/chemical oxygen demand (BOD/COD), trace quantities of biodiesel, glycerin and FFA. The methanol coming straight from the drying process has fairly large amounts of water and other materials, and the issues discussed above will result from its usage in biodiesel production.
One of the proven and effective ways to reduce water and other impurities in methanol is distilling the used or wet methanol to between 99.7 and 99.9 percent pure methanol. When considering systems to purify methanol, consider the total installed cost, operational cost, input variance (water-to-methanol to other material ratio), output quality and any waste or losses.
When water is present in the reaction, it generally manifests itself through excessive soap production. The soaps of saturated fatty acids tend to solidify at ambient temperatures, so a reaction mixture with excessive soap may gel and form a semi-solid mass that is very difficult to recover.
Some water in the system can be tolerated, because R-O- is a stronger base than OH-, so the transesterification reaction occurs at a higher rate than the saponification of glycerol, leading to FFAs. As a result of the slow reaction rate, only very minor amounts of FFAs are formed during transesterification if the reaction is free of water at the beginning.
Proceeding with transesterification with a water level in methanol higher than 500 parts per million not only leads to an undesirable byproduct of FFA, but also makes the fuel hard to pass the ASTM specifications for water and sediments, acid number and oxidation stability.
Prior to the process reactions, the obvious source of water is the feedstock. Care should be taken to make sure the feedstock has as little water possible, since no one wants to pay for water and then use precious energy drying the oil to get rid of it. Also, the premixed liquid methanol and catalyst bought should be checked for water content to make sure it has a negligible amount of moisture. Prior to the delivery, if the oil, methanol and the catalyst from vendors stay out in the open for very long, they tend to absorb water, so any quality checks have to be performed before unloading the material.
During the reactions, the major source of water in the transesterification reaction is the water carryover in the impure methanol from esterification and prior transesterification reactions (if the methanol is not purified).
Drying biodiesel and glycerin off of methanol is critical for meeting ASTM specs and to transport glycerin. Due to this drying process, used or wet methanol will consist of water with high biological oxygen demand/chemical oxygen demand (BOD/COD), trace quantities of biodiesel, glycerin and FFA. The methanol coming straight from the drying process has fairly large amounts of water and other materials, and the issues discussed above will result from its usage in biodiesel production.
One of the proven and effective ways to reduce water and other impurities in methanol is distilling the used or wet methanol to between 99.7 and 99.9 percent pure methanol. When considering systems to purify methanol, consider the total installed cost, operational cost, input variance (water-to-methanol to other material ratio), output quality and any waste or losses.
Advertisement
Advertisement
Advertisement
Advertisement
Upcoming Events





