Preventing Production Problems in the Lab


April 18, 2016
BY Trish Meek and Barbara Van Cann
Reduced oil prices have been bad news for oil industry profits. It hasn’t been easy for renewables, either. Despite dampened enthusiasm for renewables overall, innovation continues in areas such as biofuel production.
In late March, United Airlines announced that it will use biofuel to help power flights running between Los Angeles and San Francisco, with future plans to expand to all flights operating out of LAX. A small step indeed, but a positive sign that biofuel production remains viable for the future.
Now that United has publicly committed to biofuels, it must be able to rely on a steady Supply. In this case, it will come from a Los Angeles refinery operated by AltAir Fuels. The total mixture will be 30 percent biofuel, sourced from feedstocks that include algae, and 70 percent traditional jet fuel.
The United announcement is part of a groundswell of activity across the transportation sector. From air to sea, to rail to over-the-road, major consumers of fuel are eyeing more affordable solutions that also comply with mandates related to clean air. The view appears positive, and the biofuels industry should be and is thinking about a future of higher production volume and greater quality. That means production efficiency and productivity, which puts biofuel labs back on the spot to demonstrate their critical enterprise value.
As biofuel producers refocus on a more optimistic future, they will turn their attention back to the complexity of their processes. With the potential for increased demand comes the increased risk of production failure. Batch failure is magnified when production accelerates, so labs will increasingly rely on new analytical technologies and new software solutions that enable them to more closely monitor, analyze and report. This ranges from gas chromatography (GC), ion chromatography and fourier transform infrared (FTIR) spectroscopy to inductively coupled plasma mass spectrometry (ICP-MS), all of which generate volumes of data.
Volumes of data can soon lead to mountains of challenges, from daily lab operations to more complex pattern recognition that could expose risks, such batch failure. Without a solution such as a laboratory information management system (LIMS), these mountains quickly become impossible to summit.
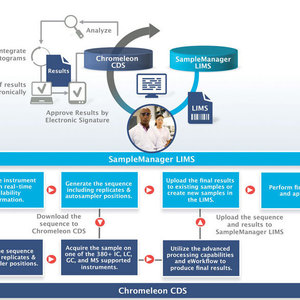
The LIMS brings discipline to an environment with many complex, moving parts. Enterprise-level LIMS, designed for integration across the lab and with other enterprise systems, can seamlessly connect with and work in harmony with other systems, such as a chromatography data system (CDS). In the next-generation biofuels lab, a CDS is critical to ensuring the quality of biofuels, which has everything to do with adherence to required gas and ion chromatographic methods.
As modern biofuel laboratories select equipment that is most suitable for specific analyses, they commonly choose from a variety of chromatography instruments from different manufacturers. Using a manufacturer-specific CDS for each instrument can lead to complications regarding efficiency, data handling, training, validation and compliance. The selection of a CDS with multivendor instrument control, including acquisition and data handling of MS instruments, overcomes these challenges. In addition, data storage and handling can be decoupled from instrument control and centralized, a so-called enterprise of the client-server system, increasing the security and accessibility of the data. With all data from instruments in the same format and one common report for results, regardless of the instrument that was used to generate them, the efficiency gains and productivity increases are considerable.
A major requirement in production laboratories is ensuring the highest instrument uptime to produce results at every time of day. Especially when running in a client-server system, keeping the laboratory fully up and running during a network outage, planned or not, is a big challenge. Therefore, a CDS should allow operation independent of the network, so that even when the network is down, the CDS keeps instruments running, data accessible for processing, and even allows creation and running of new sequences, ensuring 24/7 laboratory uptime.
By guiding technicians through the execution of analytical methods, the CDS drives the laboratory processes related to all aspects of a chromatographic analysis. Some CDS provide an even higher level of automation by encapsulating all of the unique aspects of a chromatography workflow, like instrument control and data processing parameters, correct injection order and reporting parameters, and guiding the operator through the minimal number of steps required to run it. The operator simply selects an instrument, specifies the number of samples and the starting vial position in the autosampler, and begins the analysis. The software then runs the chromatograph, processes the data, and produces final results.
Take, for example, ASTM D6584 and EN 14105, the main quantitative quality control methods for the determination of glycerol and glycerides in pure biodiesel by GC. When biodiesel (B100) is derived from vegetable oils, such as sunflower and palm oil, glycerol is created as a byproduct. Mono-, di- and triglycerides, created as intermediates or unreacted starting material, also occur. These methods test for the presence of glycerol and glycerides in the final product, which is important because of their negative impact on fuel efficiency and engine performance.
While both of these analyses differ in small ways, there are some common elements. Both methods require complex and time-consuming sample and calibration standard preparation. Samples are run in duplicate and compared to determine analytical precision. With only a few clicks in the CDS, users can create and start a run and data analysis according to the requirements in the ASTM methods is automatically performed.
The next step in the chromatography process, data processing and evaluation, is the most time-consuming step. Even with all data processing parameters correctly set up in the automated chromatography workflow, technicians need to decide if a sample requires re-analyzing, based on the results. Advanced features, such as intelligent run control, allow users to setup system suitability tests with pass/fail criteria, testing for acceptance criteria of the calibrations, checks and samples. The software can then respond to the outcome of these tests, such as failed sample replicates or standards, and without user intervention, take predefined, immediate action, such as reinjecting the samples, performing a dilution or aborting the run. As this can even happen overnight, productivity is increased and a source for errors removed.
Final results can be sent directly to the LIMS. A seamless integration between the CDS and LIMS ensures a quick response to all quality results. Any samples that fail to meet the specifications established by ASTM and EN are appropriately flagged as out of specification in the LIMS and preventative action can be taken. This level of automation across the laboratory process ensures product quality and boosts laboratory productivity.
Conclusion
A LIMS is a proven workhorse in the biofuels industry, especially because it is uniquely suited to highly distributed lab environments with multiple instrument platforms, workflows and standard operating procedures. Although instruments perform discrete tasks and generate data for specific purposes, data integration is vital and a LIMS is critical to achieving an end-to-end flow of information across the lab and across all processes. To ensure efficient, safe and profitable biofuels production, labs must be able to support continuous process monitoring and manage the data outputs in a way that is useful to stakeholders across the enterprise when, where and how they need it.
Third-generation biofuel production processes are dynamic and complex. It can take weeks for feedstock to break down, and finding a problem at the end is costly. Labs must be able to use even more sensitive analytical instruments and multilayer software infrastructure that enables instrument-, lab- and enterprise-level insight and decision-making across the production process.
Author: Trish Meek
Senior Manager, Product Marketing Informatics
& Chromatography Software
Thermo Fisher Scientific
Co-Author: Barbara van Cann
Software Product Marketing Specialist, Informatics and Chromatography Software Company
Thermo Fisher Scientific
www.thermofisher.com
Advertisement
Advertisement
Advertisement
Advertisement
Upcoming Events





