Year of the SCR System


January 19, 2010
BY Ron Kotrba
For diesel exhaust system suppliers, the past several years have been consumed by development and fine-tuning of emission control technologies so that original equipment manufacturers (OEM) can meet very strict U.S. EPA regulations on particulate matter and nitrogen oxides (NOx). That huge body of work culminates in the 2010 production year as unprecedented design and function result in ultra-sophisticated commercial systems. Liquid urea and a storage tank, injectors, mixers, catalysts and sensors are just some of the items many aftertreatment systems utilize to convert NOx into nitrogen and water. Upstream from NOx control in many designs is a diesel particulate filter (DPF), which traps virtually all of the black soot typically associated with diesel trucks. Before the DPF, most systems have a diesel oxidation catalyst (DOC) to oxidize the hydrocarbons.
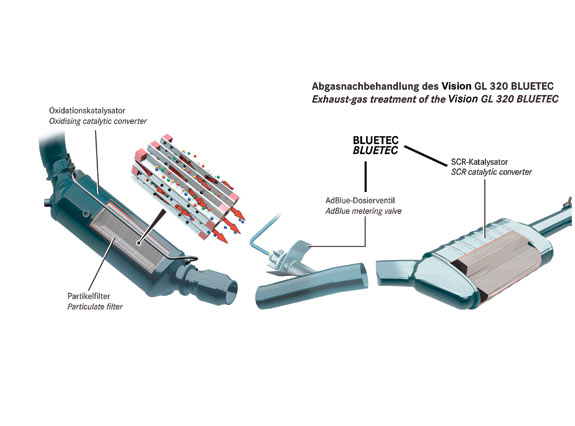
Much like a catalytic converter for gasoline systems, or a DOC, the body of the selective catalytic reduction (SCR) catalyst is a cylindrical honeycomb-like substrate with an extremely high surface area through which exhaust gases can flow encountering minimal backpressure. The material can be copper zeolite or iron zeolite. In Europe, vanadium pentoxide is used. A catalytic wash coat is applied to the body of the substrate. Depending on exhaust temperatures, urea dosing occurs via an injector and mechanical mixer in the exhaust to help atomize the fluid and disperse it evenly across the inlet face of the catalyst. The catalyst can also store a certain amount of ammonia, which, in certain conditions, can temporarily preclude the need to dose more fluid until the stored amount decreases. In a chemical reaction with the exhaust heat, the urea is transformed to ammonia and byproducts such as HNCO. The exhaust passes through the catalyst and reactions take place all along its surface, changing NOx into nitrogen and H2O.
As sophisticated as this technology might be, it is certainly not foolproof. Even though commercial production is underway, several potentially serious issues are being investigated, which, if not satisfactorily settled, could cost OEMs millions of dollars in warranty payouts-and that's without biodiesel in the picture.
Potential Issues Unrelated to Biodiesel
While some DPF regeneration schemes have issues with late post-injection and fuel dilution in the engine oil crankcase, SCR technology is prone to its own types of technological glitches. For one, the issue of ammonia slip is a common concern. Ammonia slip is when unreacted ammonia, derived from the injected urea, exits the tailpipe into the atmosphere. There are no ammonia tailpipe-out limits for mobile sources today, but this may change. Many aftertreatment designs include what's called a slip catalyst after the SCR catalyst to, for example, reduce ammonia slip from 50 parts per million (ppm) to 5 ppm. Not only can unreacted ammonia escape, but a reverse reaction may occur in which ammonia, nitrogen and water can recombine to produce tailpipe-out NOx-precisely what the system is designed to eliminate.
According to an engineer for an OEM supplier, another potential issue with SCR systems is the buildup of urea deposits on the catalyst surface. "It's one of the big issues," the source says. "It's an indirect failure when these spots of concentrated urea can block the passage of exhaust gas either on or around the mixers, or at the injection mount, or downstream even at the face of the catalyst. The best way to avoid this is to make sure the exhaust pipe and everything from the point of urea injection down through the catalyst is insulated well, at minimum with air gap insulation. One reason for this is to keep the heat in, but more importantly, to elevate the temperatures of the inner wall and the mixer." Essentially, the more insulated the system, the more likely urea deposits will be avoided.
There is also the potential for hydrocarbon absorption on the SCR catalyst, depending on how the systems are designed. Rasto Brezny, deputy director for the Manufacturers of Emissions Control Association, says, "This is a topic that came up a number of years ago from testing where the SCR was upstream of the DPF, which is not a conventional way of doing things these days. In most light duty and heavy duty designs, it's the DOC followed by the DPF with the SCR downstream. The DOC and DPF are very effective at oxidizing hydrocarbons. I know our members are aware of this issue, and since it arose and appeared in the scientific literature, most systems have been designed to minimize that interaction."
Aaron Williams, engineer with National Renewable Energy Laboratory in Golden, Colo., says the iron zeolite catalysts have demonstrated many more issues with hydrocarbon absorption. "The copper zeolite seems to tolerate the hydrocarbons better," Williams tells Biodiesel Magazine. In the event that hydrocarbon loading is an issue for a particular system design, at best it could mean blocking the reaction site disallowing catalysis to take place in certain regions of the substrate; at worst, catastrophic failure of the catalyst from an exothermic reaction if all the hydrocarbons burn off at once.
Speculation has arisen as to whether the urea, also called diesel exhaust fluid (DEF), infrastructure will be in place soon enough to meet expected demand. SCR systems will not fail per se if the DEF is not replenished immediately, but it will be out of emissions compliance and, moreover, the system is designed to go through what's called a derate strategy, which involves depowering the engine and having terrible fuel economy-incentive for the driver to fill up on the proper fluid. But putting uncertified fluid such as water in the tank, as to fool the low-level DEF indicator, could "definitely drive some warranty issues," one source says. "If the driver is injecting water or fuel so he can keep driving and his low-level indicator shows a full tank, this could cause the SCR cat to be irreparably damaged, including injectors and everything else. That's why they're incorporating NOx sensors into the system so, even if you have some fluid in your tank, are you truly reducing NOx? And second, there may be urea quality sensors in the tank to look at the pH levels of urea to determine if it's an appropriate pH."
One of the lesser known potential problems that could arise from SCR technology is unintentional formation of dioxins, very toxic compounds. "All the conditions are ripe for dioxin formation when you look at the things you need to create dioxins: copper, heat, chlorine and water," the source says. "This has been an ongoing thing with EPA. MECA is putting together a bunch of new tests to try and disprove the formation of dioxins in diesel aftertreatment systems. So that's in process now. From our standpoint, if we're collecting all this data and we're finding negligible formation of dioxins, we shouldn't continue wasting everyone's time and money and just call it good." The concern is with the copper zeolite catalysts, not the iron-based variety. "Worst case scenario, we might see a quadruple in dioxin formation with copper SCR, which might change the direction of some system designs, including Cummins, since they've claimed copper is their prime catalyst path." Some systems, however, could employ one iron and one copper catalyst-a "two-brick strategy."
Enter Biodiesel
The most researched, and really the only known, potential problem with using biodiesel blends in diesel SCR systems involves trace metals left in the biodiesel from processing-sodium or potassium, and calcium or magnesium. "Over time these metals, once they go through the combustion process in the engine, they end up in the exhaust and that ash can attach itself to different aftertreatment devices, including SCR catalysts," Williams says. "And it can attack those devices. After 435,000 miles," the full useful life of aftertreatment systems for heavy duty vehicles, "you'll see a lot of ash from biodiesel-from these metals-and that could foul the catalyst." Williams says on systems where the DOC and DPF are upstream of the SCR catalyst, the chance of fouling will be reduced, but he adds that some metals from the ash, when in a vapor state, can still pass through the DPF to enter the SCR catalyst. He also says some light duty applications have the SCR system upstream of the DPF, in which cases metals entering the SCR is much more of a concern.
The limit on these ashes in ASTM D6751 is 5 ppm for calcium and magnesium, and 5 ppm for sodium and potassium, but most B100 on the market comes nowhere near those amounts. "Based on NREL's fuel quality surveys, you hardly ever find detectable levels of these metals in the fuel," Williams says. The detectable limit is 1 ppm. "So we're talking about down to the parts-per-billion (ppb) range as far as at what levels these trace metals are in quality biodiesel. But if you do a back-of-the-envelope calculation and look at the current spec for allowable metals in biodiesel, a combined 10 ppm, and if you consider a B20 blend, the amount of accumulated ash that could lead to equivalent to the amount of ash you would get from lubricant oil over time, roughly speaking." He says what is really needed is a new method to detect these metals in the ppb range. This would be necessary to eventually lowering the limit in the quality spec, and adjusting the spec may be exactly the impetus to encourage more OEMs' endorsement of B20.
Lou Wenzler, on-highway marketing communications director for Cummins, says all of Cummins' on-highway engines for 2010 are B20 compatible. "We're fine with biodiesel," Wenzler says. "There are no issues for Cummins on using B20 in all our 2010 systems, specifically SCR. We're B20 compatible today, and tomorrow." Cummins does have a long, thorough history in testing biodiesel and understanding its performance in new systems, so it appears this greater understanding justifies the company's unconditional acceptance of ASTM quality B20.
According to Williams, though, this matter of alkali metals is a show-stopper issue for most OEMs. This is why NREL is conducting a study supported by all the major OEMs and the National Biodiesel Board, the Engine Manufacturers Association and MECA, to take an SCR system that is connected to an engine running on B20, with metals content at the threshold limit, to its full useful life of 435,000 miles to determine exactly what effect this truly has on the catalyst. "If our tests show that limit is too high and it's detrimental to the SCR cat or the other devices, we'd go to ASTM and recommend that the limit be lowered, which couldn't be too much of a challenge considering most of the biodiesel comes in much lower than the current limits," Williams says. Other than this possible long-term concern over using biodiesel blends with SCR systems, Williams says there aren't really any other potentially negative interactions to speak of.
NREL recently conducted another study, this time with Ford Motor Co., to investigate how biodiesel might play a role in the issue of hydrocarbon absorption. "Since biodiesel hydrocarbons are different than petroleum hydrocarbons, we wanted to see how biodiesel might affect this," Williams tells Biodiesel Magazine. They tested straight B100 in this study. "One thing we found was you get less hydrocarbon absorption because the amount of engine-out hydrocarbons is lower with biodiesel. Another thing we found was that if you saturate the catalyst with hydrocarbons from biodiesel, they come off a lot easier than hydrocarbons from ULSD do. We're doing follow up studies to see exactly why that's the case. But the basic conclusion is that the hydrocarbon poisoning issue for SCR catalysts is no worse at least from using biodiesel."
Ron Kotrba is the editor of Biodiesel Magazine. Reach him at (701) 738-4942 or rkotrba@bbiinternational.com.
Much like a catalytic converter for gasoline systems, or a DOC, the body of the selective catalytic reduction (SCR) catalyst is a cylindrical honeycomb-like substrate with an extremely high surface area through which exhaust gases can flow encountering minimal backpressure. The material can be copper zeolite or iron zeolite. In Europe, vanadium pentoxide is used. A catalytic wash coat is applied to the body of the substrate. Depending on exhaust temperatures, urea dosing occurs via an injector and mechanical mixer in the exhaust to help atomize the fluid and disperse it evenly across the inlet face of the catalyst. The catalyst can also store a certain amount of ammonia, which, in certain conditions, can temporarily preclude the need to dose more fluid until the stored amount decreases. In a chemical reaction with the exhaust heat, the urea is transformed to ammonia and byproducts such as HNCO. The exhaust passes through the catalyst and reactions take place all along its surface, changing NOx into nitrogen and H2O.
As sophisticated as this technology might be, it is certainly not foolproof. Even though commercial production is underway, several potentially serious issues are being investigated, which, if not satisfactorily settled, could cost OEMs millions of dollars in warranty payouts-and that's without biodiesel in the picture.
Potential Issues Unrelated to Biodiesel
While some DPF regeneration schemes have issues with late post-injection and fuel dilution in the engine oil crankcase, SCR technology is prone to its own types of technological glitches. For one, the issue of ammonia slip is a common concern. Ammonia slip is when unreacted ammonia, derived from the injected urea, exits the tailpipe into the atmosphere. There are no ammonia tailpipe-out limits for mobile sources today, but this may change. Many aftertreatment designs include what's called a slip catalyst after the SCR catalyst to, for example, reduce ammonia slip from 50 parts per million (ppm) to 5 ppm. Not only can unreacted ammonia escape, but a reverse reaction may occur in which ammonia, nitrogen and water can recombine to produce tailpipe-out NOx-precisely what the system is designed to eliminate.
According to an engineer for an OEM supplier, another potential issue with SCR systems is the buildup of urea deposits on the catalyst surface. "It's one of the big issues," the source says. "It's an indirect failure when these spots of concentrated urea can block the passage of exhaust gas either on or around the mixers, or at the injection mount, or downstream even at the face of the catalyst. The best way to avoid this is to make sure the exhaust pipe and everything from the point of urea injection down through the catalyst is insulated well, at minimum with air gap insulation. One reason for this is to keep the heat in, but more importantly, to elevate the temperatures of the inner wall and the mixer." Essentially, the more insulated the system, the more likely urea deposits will be avoided.
There is also the potential for hydrocarbon absorption on the SCR catalyst, depending on how the systems are designed. Rasto Brezny, deputy director for the Manufacturers of Emissions Control Association, says, "This is a topic that came up a number of years ago from testing where the SCR was upstream of the DPF, which is not a conventional way of doing things these days. In most light duty and heavy duty designs, it's the DOC followed by the DPF with the SCR downstream. The DOC and DPF are very effective at oxidizing hydrocarbons. I know our members are aware of this issue, and since it arose and appeared in the scientific literature, most systems have been designed to minimize that interaction."
Aaron Williams, engineer with National Renewable Energy Laboratory in Golden, Colo., says the iron zeolite catalysts have demonstrated many more issues with hydrocarbon absorption. "The copper zeolite seems to tolerate the hydrocarbons better," Williams tells Biodiesel Magazine. In the event that hydrocarbon loading is an issue for a particular system design, at best it could mean blocking the reaction site disallowing catalysis to take place in certain regions of the substrate; at worst, catastrophic failure of the catalyst from an exothermic reaction if all the hydrocarbons burn off at once.
Speculation has arisen as to whether the urea, also called diesel exhaust fluid (DEF), infrastructure will be in place soon enough to meet expected demand. SCR systems will not fail per se if the DEF is not replenished immediately, but it will be out of emissions compliance and, moreover, the system is designed to go through what's called a derate strategy, which involves depowering the engine and having terrible fuel economy-incentive for the driver to fill up on the proper fluid. But putting uncertified fluid such as water in the tank, as to fool the low-level DEF indicator, could "definitely drive some warranty issues," one source says. "If the driver is injecting water or fuel so he can keep driving and his low-level indicator shows a full tank, this could cause the SCR cat to be irreparably damaged, including injectors and everything else. That's why they're incorporating NOx sensors into the system so, even if you have some fluid in your tank, are you truly reducing NOx? And second, there may be urea quality sensors in the tank to look at the pH levels of urea to determine if it's an appropriate pH."
One of the lesser known potential problems that could arise from SCR technology is unintentional formation of dioxins, very toxic compounds. "All the conditions are ripe for dioxin formation when you look at the things you need to create dioxins: copper, heat, chlorine and water," the source says. "This has been an ongoing thing with EPA. MECA is putting together a bunch of new tests to try and disprove the formation of dioxins in diesel aftertreatment systems. So that's in process now. From our standpoint, if we're collecting all this data and we're finding negligible formation of dioxins, we shouldn't continue wasting everyone's time and money and just call it good." The concern is with the copper zeolite catalysts, not the iron-based variety. "Worst case scenario, we might see a quadruple in dioxin formation with copper SCR, which might change the direction of some system designs, including Cummins, since they've claimed copper is their prime catalyst path." Some systems, however, could employ one iron and one copper catalyst-a "two-brick strategy."
Enter Biodiesel
The most researched, and really the only known, potential problem with using biodiesel blends in diesel SCR systems involves trace metals left in the biodiesel from processing-sodium or potassium, and calcium or magnesium. "Over time these metals, once they go through the combustion process in the engine, they end up in the exhaust and that ash can attach itself to different aftertreatment devices, including SCR catalysts," Williams says. "And it can attack those devices. After 435,000 miles," the full useful life of aftertreatment systems for heavy duty vehicles, "you'll see a lot of ash from biodiesel-from these metals-and that could foul the catalyst." Williams says on systems where the DOC and DPF are upstream of the SCR catalyst, the chance of fouling will be reduced, but he adds that some metals from the ash, when in a vapor state, can still pass through the DPF to enter the SCR catalyst. He also says some light duty applications have the SCR system upstream of the DPF, in which cases metals entering the SCR is much more of a concern.
The limit on these ashes in ASTM D6751 is 5 ppm for calcium and magnesium, and 5 ppm for sodium and potassium, but most B100 on the market comes nowhere near those amounts. "Based on NREL's fuel quality surveys, you hardly ever find detectable levels of these metals in the fuel," Williams says. The detectable limit is 1 ppm. "So we're talking about down to the parts-per-billion (ppb) range as far as at what levels these trace metals are in quality biodiesel. But if you do a back-of-the-envelope calculation and look at the current spec for allowable metals in biodiesel, a combined 10 ppm, and if you consider a B20 blend, the amount of accumulated ash that could lead to equivalent to the amount of ash you would get from lubricant oil over time, roughly speaking." He says what is really needed is a new method to detect these metals in the ppb range. This would be necessary to eventually lowering the limit in the quality spec, and adjusting the spec may be exactly the impetus to encourage more OEMs' endorsement of B20.
Lou Wenzler, on-highway marketing communications director for Cummins, says all of Cummins' on-highway engines for 2010 are B20 compatible. "We're fine with biodiesel," Wenzler says. "There are no issues for Cummins on using B20 in all our 2010 systems, specifically SCR. We're B20 compatible today, and tomorrow." Cummins does have a long, thorough history in testing biodiesel and understanding its performance in new systems, so it appears this greater understanding justifies the company's unconditional acceptance of ASTM quality B20.
According to Williams, though, this matter of alkali metals is a show-stopper issue for most OEMs. This is why NREL is conducting a study supported by all the major OEMs and the National Biodiesel Board, the Engine Manufacturers Association and MECA, to take an SCR system that is connected to an engine running on B20, with metals content at the threshold limit, to its full useful life of 435,000 miles to determine exactly what effect this truly has on the catalyst. "If our tests show that limit is too high and it's detrimental to the SCR cat or the other devices, we'd go to ASTM and recommend that the limit be lowered, which couldn't be too much of a challenge considering most of the biodiesel comes in much lower than the current limits," Williams says. Other than this possible long-term concern over using biodiesel blends with SCR systems, Williams says there aren't really any other potentially negative interactions to speak of.
NREL recently conducted another study, this time with Ford Motor Co., to investigate how biodiesel might play a role in the issue of hydrocarbon absorption. "Since biodiesel hydrocarbons are different than petroleum hydrocarbons, we wanted to see how biodiesel might affect this," Williams tells Biodiesel Magazine. They tested straight B100 in this study. "One thing we found was you get less hydrocarbon absorption because the amount of engine-out hydrocarbons is lower with biodiesel. Another thing we found was that if you saturate the catalyst with hydrocarbons from biodiesel, they come off a lot easier than hydrocarbons from ULSD do. We're doing follow up studies to see exactly why that's the case. But the basic conclusion is that the hydrocarbon poisoning issue for SCR catalysts is no worse at least from using biodiesel."
Ron Kotrba is the editor of Biodiesel Magazine. Reach him at (701) 738-4942 or rkotrba@bbiinternational.com.
Advertisement
Advertisement
Upcoming Events





