The Chemical Kinetics of Glycerolysis


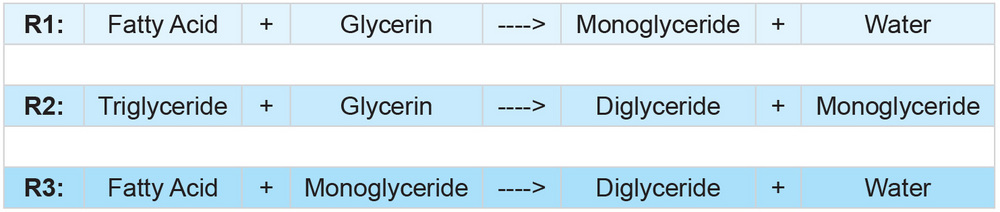
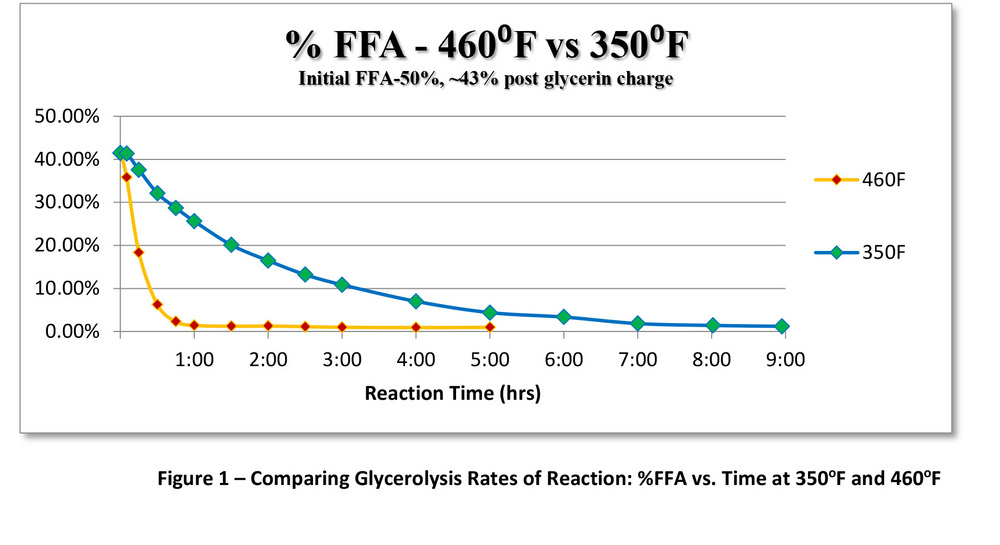
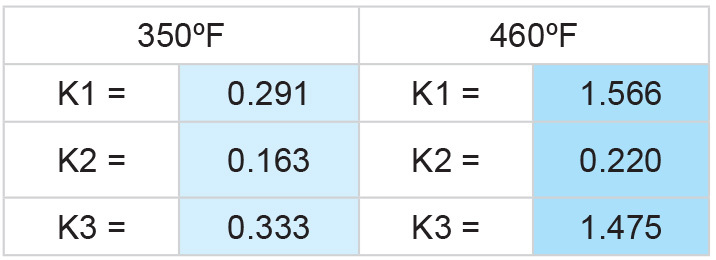

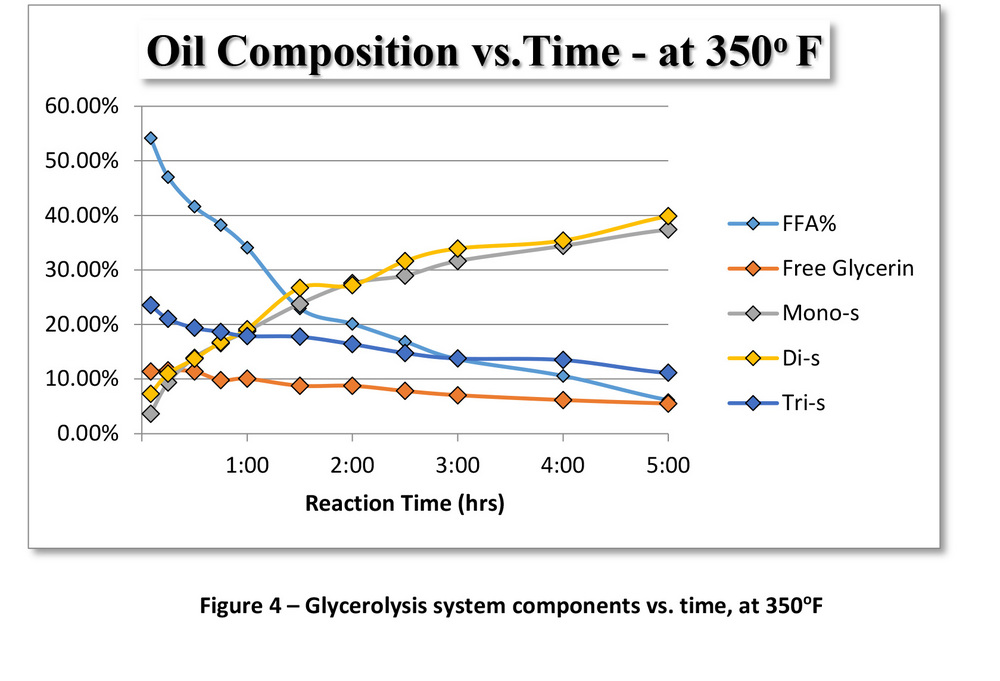

PHOTO: SUPERIOR PROCESS TECHNOLOGIES LLC
May 15, 2014
BY Erik Anderson
Advertisement
Advertisement
Related Stories
Rep. Randy Feenstra, R-Iowa, and five of his colleagues on the U.S. House Ways and Means Committee on Nov. 18 announced they are seeking additional information from relevant stakeholders on biofuel tax policy. Reponses must be submitted by Dec. 13.
Corteva Inc. has announced plans to form a joint venture with bp focused on feedstock production for SAF. The company plans to grow proprietary Corteva mustard seed, sunflower and canola feedstocks well-suited for SAF production.
DOE announces $20.2 million in projects to advance development of mixed algae for biofuels and bioproducts
The U.S. Department of Energy on Nov. 14 announced $20.2 million in funding for 10 university and industry projects spanning seven states to advance mixed algae development for low-carbon biofuels and bioproducts.
The USDA released its latest Crop Production report on Nov. 8, reporting reduced forecasts for 2024 soybean production and yields. Both production and yields, however, are up significantly when compared to last year.
The USDA maintained its forecast for 2024-’25 soybean oil use in biofuel production in its latest World Agricultural Supply and Demand Estimates report, released Nov. 8. The U.S. season-average price for soybean oil was revised up 1 cent per pound.





