Ion Exchange Resin as Dry Wash Media



March 12, 2013
BY Rod Yawn
Ion exchange resin was introduced in the biodiesel industry as a dry-wash media. Now, it is being used for a number of other applications in biodiesel plants, including esterification catalysts, media to improve cold soak filtration test performance and glycerin purification. Ironically, ion exchange resin is increasingly being utilized in water-wash plants to decatonize wash water, and to polish and dry the finished fuel.
The term dry wash is an oxymoron; dry wash is a term commonly used to define any biodiesel purification process that does not utilize water, including everything from absorbents to ion exchange resin. More than 90 percent of the biodiesel gallons that are produced with the dry-wash process use ion exchange resin (DW-Resin). Fortunately, in the years since it was introduced, the cost of DW-Resin has come down and resin quality has improved. Use costs for DW-Resin now range from 2 to 4 cents per gallon of biodiesel treated. In comparison, the most commonly utilized absorbent, magnesium silicate, has a use cost of 9 to 14 cents per gallon.
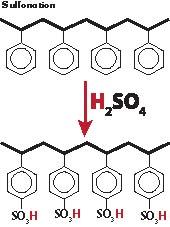
The base structure DW-Resin is a copolymer of styrene cross-linked with divinylbenzene. This compound is extremely tough. The next step in the manufacturing process attaches millions of ion exchange sites to the copolymer matrix through a process called sulfonation.
These strongly charged sites will exchange a hydrogen ion for other cations (i.e., Na++, K++ and Ca++). Of course, this exchange can result in soap being split by the removal of sodium or potassium from the molecule. It is important to note, however, that this is the least desirable activity the DW-Resin can perform. Of greater importance is the powerful attraction that the resin exhibits for polar compounds, particularly glycerin. This attraction is due to the demand for hydration by the charged sites within the resin bead. The absorption of glycerin, or other polar compounds like water and methanol, does not exhaust the ion exchange capacity of the resin. Glycerin absorption can be reversed by replacing the glycerin with another polar compound like methanol, in the wash process. Theoretically, the resin could go through the glycerin absorption cycle an infinite number of times. In practice, the exchange of cations from soap, or excess catalyst, reduces the absorptive capacity of the resin. Another factor that will reduce resin life is fouling by organics like unsaponifiable matter.
As we have discussed, DW-Resin can remove contaminants by ion exchange and by absorption. One of the most common misconceptions is that the resin removes glycerin by holding it on the surface of the beads. Glycerin, water and methanol are actually pulled into the beads, however, causing them to swell to double their original size. The resin bed also removes a significant amount of insoluble soap (micelles), glycerin, and unsaponifiable matter by simple filtration. There are 59 million DW-Resin beads in a gallon, with a total surface area of 1,995 square feet. The surface of the resin becomes coated with soap and unsaponifiable matter. This sticky layer attracts more insoluble matter and promotes the formation of soap micelles. If the column is left in service too long, or if the loading of insoluble matter is high, the column can release a slug of contaminants.
The three ways DW-Resin removes contaminants from biodiesel—ion exchange of catalyst and soap, absorption of glycerin and water, and filtration of insoluble soap and unsaponifiable matter—are accomplished in a column. Proper design of the column and control of process flow is critical to optimum performance. Operating in a lead/lag configuration at a process flow rate of around three bed volumes per hour is optimum. The flow rate is important because the absorption of glycerin requires time. The amount of time for absorption is related to temperature, methanol concentrations and the percent capacity remaining in the resin. The ideal temperature is 115 degrees Fahrenheit. The acceptable temperature range is 90 to 140 degrees. It is often said that there must be some methanol in the process stream to make DW-Resin work. That is true, but it should be noted that there is always some methanol present in biodiesel, even when the biodiesel is within specifications. The real issue is the ratio of methanol to the level of soap and glycerin in the process stream. Absorption rates will be slow for a stream with high-glycerin/soap and low-methanol content.
Most plants operate their dry-wash columns prior to demethylation. This is a good option because it protects the demethylation system from fouling with soap and glycerin. It also allows the resin to be washed with methanol when it becomes loaded with glycerin and soap. It is becoming increasingly common for plants to install dry-wash polishing columns after demethylation. Because soap and glycerin levels are very low at that point, the resin can polish many gallons prior to reaching saturation. Frequently, plants will move the resin to one of their columns prior to demethylation after it reaches its absorption capacity. The resin is then washed with methanol and put into service.
Of course, the total cost of the dry-wash process per gallon is a key issue. A survey of the larger producers that utilize the dry-wash process indicates a wide range of gallons treated per pound of resin exhausted. The range is 100 to 220 gallons per pound. The median number is around 170 gallons per pound. The range is wide because of the variability in feedstock, dosing strategy and separation technology prior to the dry-wash columns. Another significant factor determining resin performance and longevity is the methanol washing process. The temperature of the methanol used in washing dry-wash resin is very important. Many plants store methanol outside, and end up washing resin with cold methanol. Heating the methanol will help remove congealed soap, unsaponifiables and glycerol. There are some other common mistakes that are made regarding washing columns with methanol. Recirculating the first methanol rinse through the resin will increase the amount of soap that is split by the resin. That exhausts the exchange capacity of the resin. The first rinse should be a soak-then-drain procedure. Not applying a final rinse will leave glycerin in the resin bed. The glycerin left in the bed will end up getting flushed into the process stream when the column is put back into service.
At a time when even bad feedstock is expensive, more plants are running animal fats and yellow grease. More plants are using acid esterification, many without adequate drying prior to transesterification. Soap is up. Consequently, improved separation prior to dry wash is more important than ever. Many plants were built with gravity settlers as their only separation option. This technology was adequate when the plants were running low-FFA vegetable oils. Unfortunately, gravity settlers are limited by the fact that glycerin and soap are emulsified in methyl ester during the transesterification process. Consider homogenized milk. It is simply an emulsion of water, protein and fat. Milk is homogenized by a mechanical process that involves heat and agitation to create an emulsion. Does this sound a little like your transesterification reactor? Coalescers are commonly used to break emulsions. Unfortunately, coalescers have a bad name in the biodiesel industry because they tend to foul with soap. There are some new options out there including inline electrocoalescers. There are also some strategies that will help without adding more equipment. Keeping the biodiesel temperature up in the decanter will speed settling.
Ultimately, only one thing matters more than making quality biodiesel at a profit. That, of course, is safety. A dry-wash column that is open at the top could possibly be full of methanol vapor. It is easy to forget that methanol vapor is heavier than air. A worker who is above the column, loading resin, is at risk. It is important to insure that the column is grounded and does not contain concentrated methanol vapors. A spark from any source could spell trouble.
---------------------
Author: Rod Yawn
President, ALX Enterprises LLC
615-866-0828
rod@alxent.com
Advertisement
Advertisement
Related Stories
Biodiesel capacity in the U.S. and Canada dipped slightly stable in 2024, with several renewable diesel producers reporting headwinds and lower margins alongside a drove of SAF projects in various stages of development.
The IEA’s Task 39 group has new research regarding the development and status of the sustainable aviation fuel industry.
The U.S. EPA on Nov. 16 released updated RIN data, reporting that nearly 2.11 billion RINs were generated under the RFS in October, up from 1.81 billion generated during the same month of last year.
Conestoga to host SAFFiRE cellulosic ethanol pilot plant
Conestoga Energy and SAFFiRE Renewables LLC announced on Nov. 16 their agreement for Conestoga to host SAFFiRE’s cellulosic ethanol pilot plant at Conestoga’s Arkalon Energy ethanol facility in Liberal, Kansas.
Officials at Calumet Specialty Products Partners L.P. discussed the company’s proposed plans to boost sustainable aviation fuel (SAF) production at its Montana Renewables biorefinery during third quarter earnings call, held Nov. 9.
Upcoming Events










